2020 P1 Q1 - Deducing number of d-orbitals with 4 lobes
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 1.
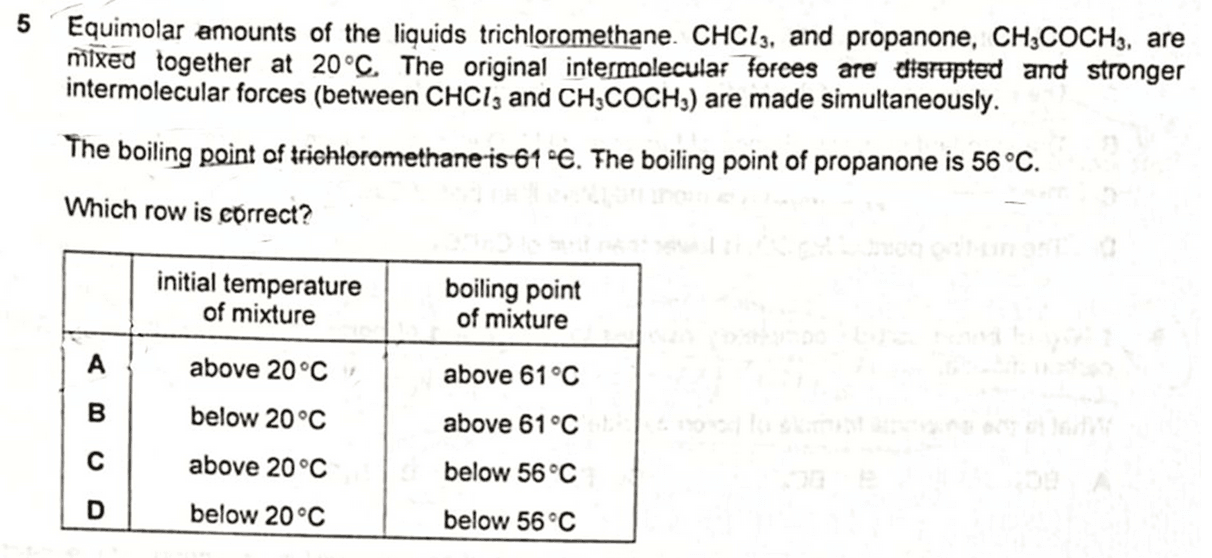
We are required to determine the number of 3d orbitals that has four lobes.
Let's recap the shape of p orbitals and d orbitals.
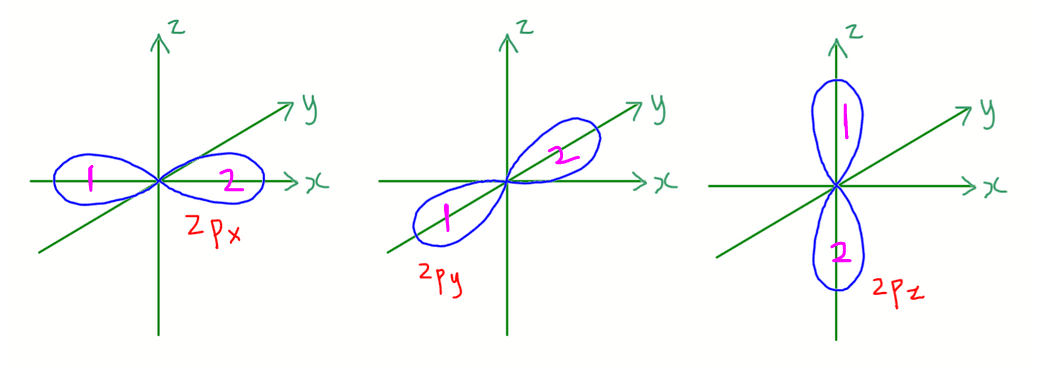
There are 3 p orbitals in each p subshell.
p orbitals are dumbbell shaped with 2 lobes.
- 2px lies along x axis
- 2py lies along y axis
- 2pz lies along z axis
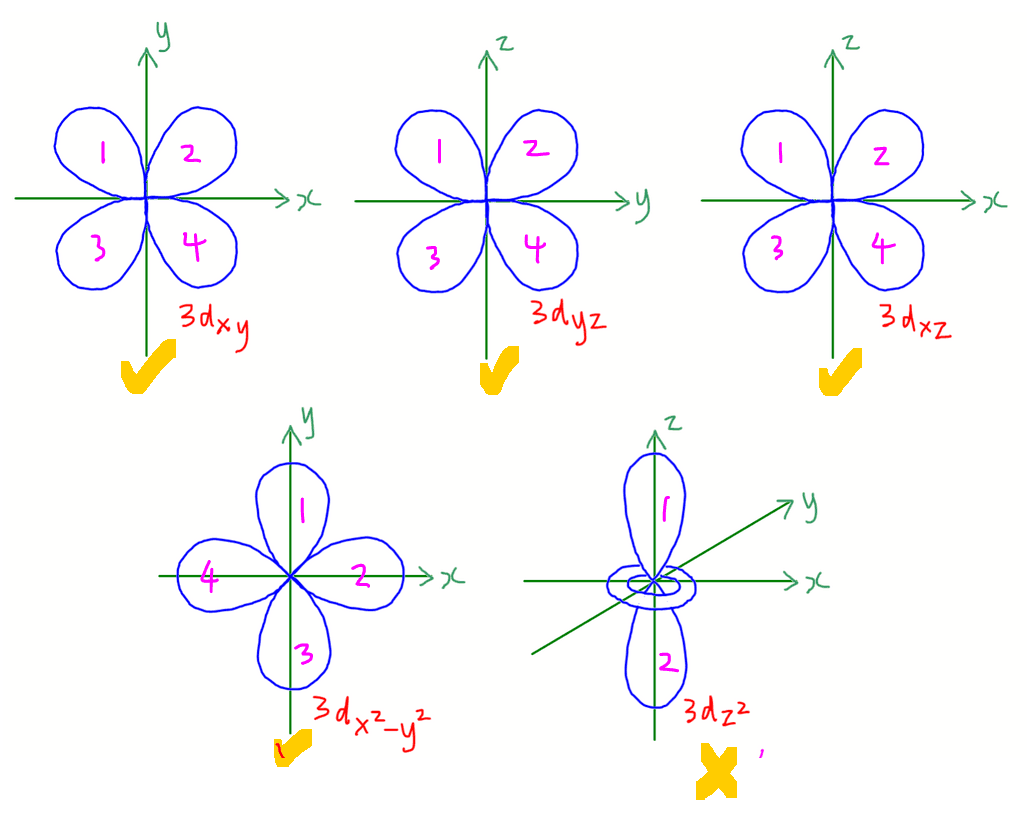
There are 5 d orbitals in each d subshell.
4 of the d orbitals are clover-leaf shaped with 4 lobes.
- 3dxy lies on xy plane
- 3dyz lies on yz plane
- 3dxz lies on xz plane
- 3dx2-y2 lies directly along x and y axis
The final d orbital 3dz2 is dumbbell shaped with a ring around the centre lying along z axis.

Therefore the answer to this question will be option C.
Topic: Atomic Structure, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
