2020 P1 Q12 - Compare Rate and Determine Volume of CO2 Formed
Let Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 12.
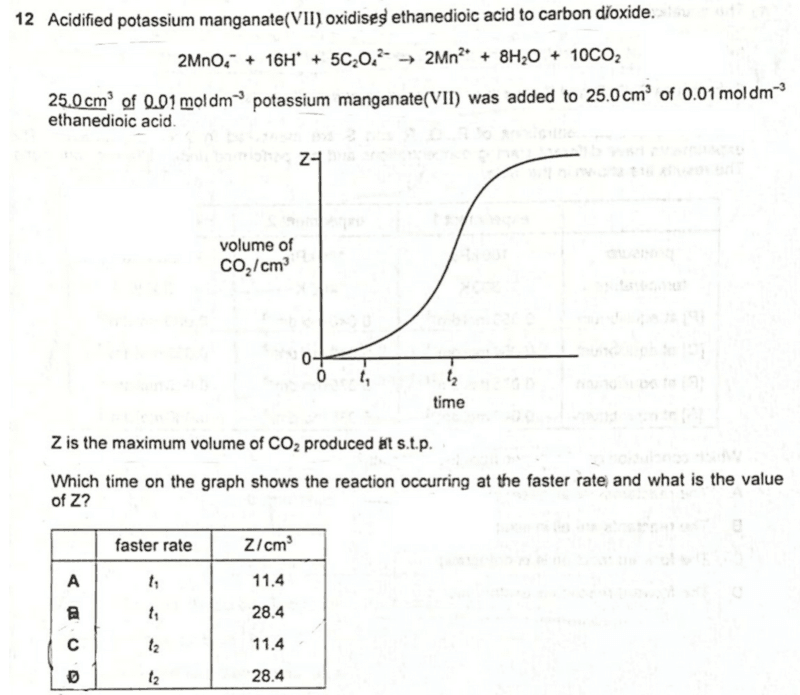
We are required to deduce which time shows the reaction at the faster rate and the value of Z, the maximum volume of CO2 produced at stp.
Let's do this part by part.
1. Compare Rate
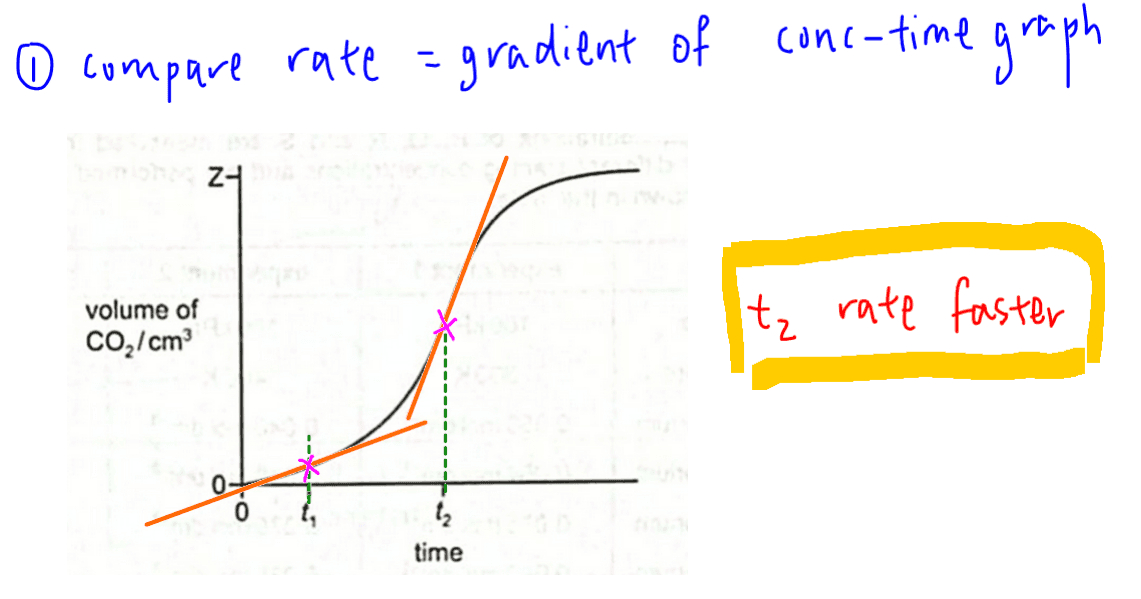
Comparing rate at different times is fairly straight forward.
Since rate is the gradient of concentration time graph, we just need to compare the slope of the graph at both times t1 and t2.
From the graph we can see clearly that the gradient of the slope at t2 is steeper, hence rate of reaction at t2 is faster.
2. Calculate Max Volume of CO2
Since the amounts of both reactants are given, we need to determine which of them is limiting.
Check out this video for a detailed discussion of deducing limiting reagent.
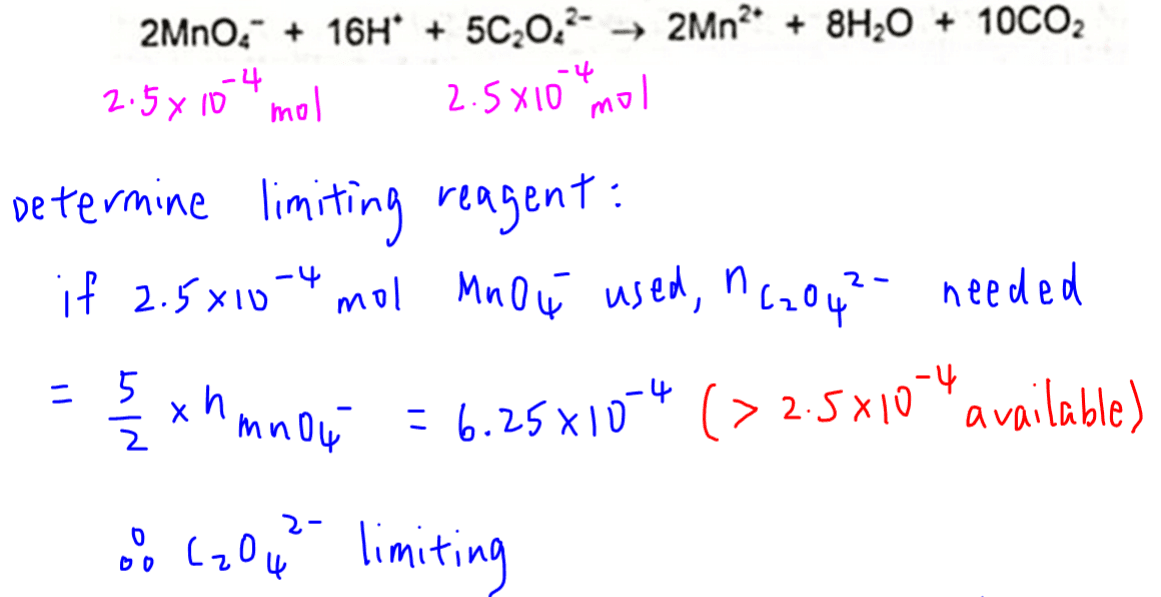
If 2.5 x 10-4 moles of MnO4- were used up, amount of C2O42- required will be 6.25 x 10-4 moles.
Since we only have 2.5 x 10-4 moles of C2O42-, there is insufficient C2O42- to react with all the MnO4-.
Hence C2O42- is limiting.
We can now use the limiting reagent to determine amount and volume of carbon dioxide formed.
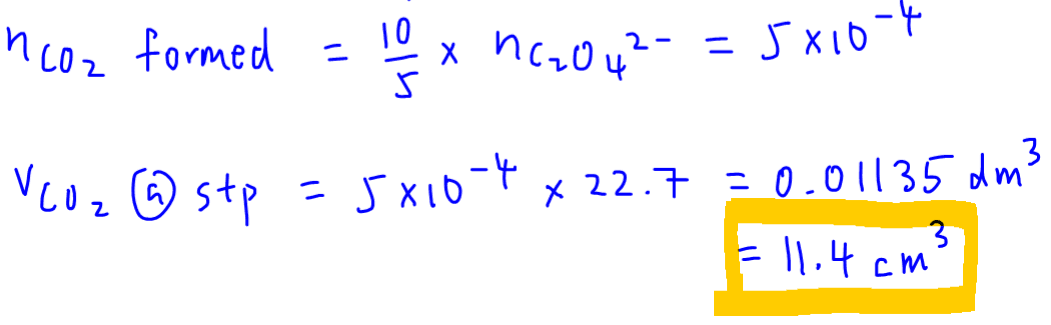
Hence 11.4 cm3 of CO2 is formed at stp.
Let's run through the options and determine our answer.

Remember the faster rate is at t2 and max volume of CO2 is 11.4 cm3.
Therefore the answer to this question will be option C.
Topic: Kinetics and Mole Concept, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
