2020 P1 Q13 - Determine Initial H2O2 Concentration for First Order Reaction
Let Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 13.

We are required to determine initial concentration of the hydrogen peroxide solution.
Since the reaction has a constant half life of 40 minutes, we know that the reaction is first order with respect to hydrogen peroxide.
Let's break this down into 3 main steps.
1. Deduce Number of Half Lifes
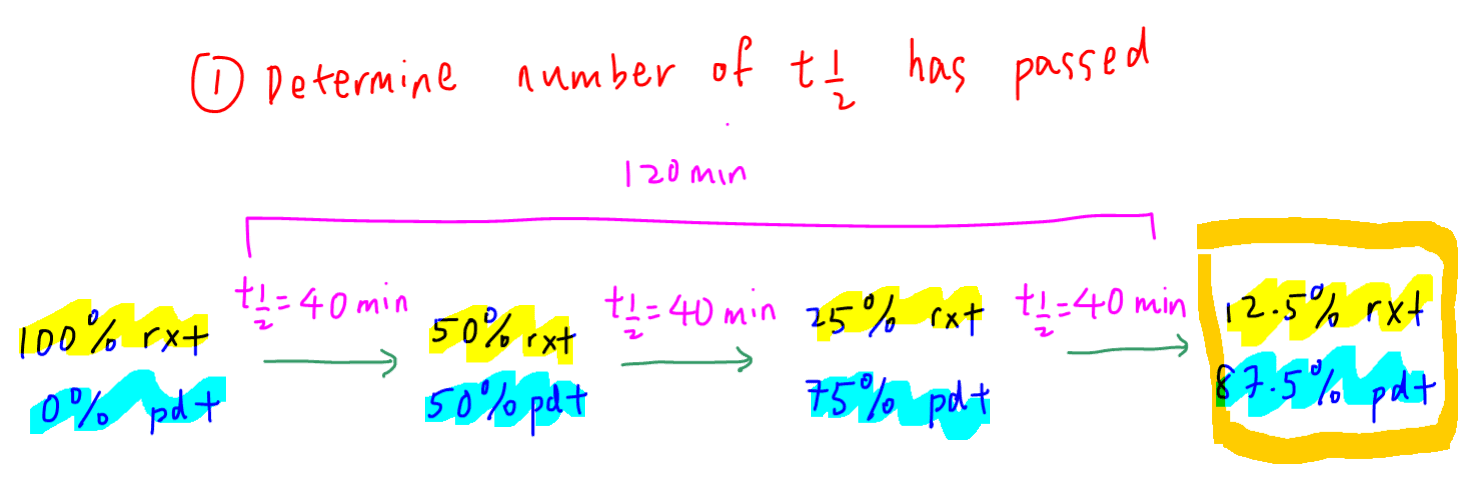
Since time taken was 120 minutes, 3 half lifes of 40 minutes each have passed.
At the end of the third half life, 12.5% of reactants is left and 87.5% of products is formed.
2. Determine Volume of O2 at End of Reaction

87.5% of products corresponds to 6.00 dm3 of O2.
Hence 100% of products will correspond to 6.857 dm3 of O2.
Therefore the maximum volume of O2 formed is 6.857 dm3.
3. Determine Concentration of H2O2

We can find the amount of O2 formed at rtp to be 0.2857 moles.
This means the amount of H2O2 reacted is 0.5714 moles.
We can finally calculate the concentration of H2O2 at 2.857 mol dm-3 or 2.9 mol dm-3 (rounded off to 2 significant figures).

Hence the answer to this question will be option D.
Topic: Kinetics, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
