2020 P1 Q17 - Describing Constitutional Isomers
Let Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 17.

We want to determine which statement is correct to describe constitutional isomers.
Let's have a recap of constitutional or structural isomerism.
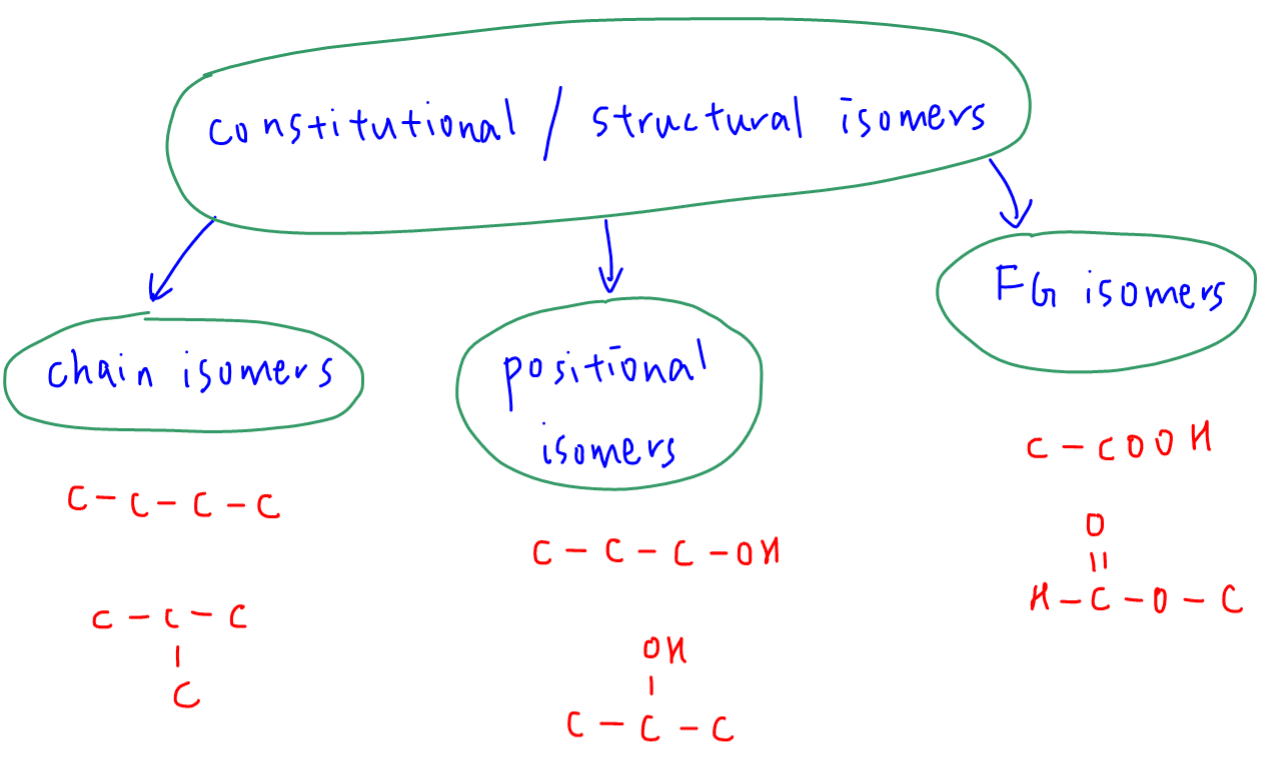
Constitutional or structural isomers have the same molecular formula but different structural formula.
In syllabus we have 3 types of structural isomers to consider.
1. Chain isomers - different carbon chain arrangements, eg butane and 2-methylpropane
2. Positional isomers - same functional group on a different carbon, eg propan-1-ol and propan-2-ol
3. Functional group isomers - different functional groups, eg ethanoic acid and ester methyl methanoate
Check out this video lesson to learn more about structural isomerism.
Let's run through each statement and see which is the best answer.
A. C4H10 has 3 constitutional isomers.
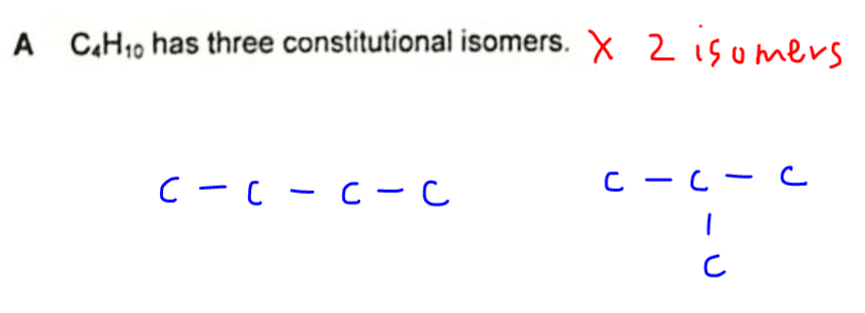
We have only 2 chain isomers possible for C4H10, hence statement A is not true.
B. But-1-ene and pent-1-ene are examples of constitutional isomers.

The number of carbons for butene and pentene are different hence they will not have the same molecular formula.
They are not isomers, hence statement B is not true.
C. Constitutional isomers may differ in their chemical properties.

Isomers are different compounds with different physical and/or chemical properties.
For example, functional group isomers ethanoic acid and methyl methanoate differ greatly in their reactivity since the functional groups are different.
Positional isomers propan-1-ol and propan-2-ol are oxidised to different products acid and ketone respectively so their chemical properties differ too.
Hence statement C is true and should be the best answer.
D. Constitutional isomers may have different molecular formulae.

Constitutional isomers must have the same molecular formula, ie same number of carbon, hydrogen, oxygen, etc.
The difference is in their structural formula.
Hence statement D is not true.
Therefore the answer to this question will be option C.
Topic: Intro to Organic Chemistry, Organic Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
