2020 P1 Q19 - Free Radical Reaction of Ozone
Let Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 19.
We want to determine which of the following statements are correct.
1. F• and •CCl2F are the major free radical products made during the initiation step.

There are only 2 types of bonds in the reactant CCl2F2: C-Cl bond and C-F bond.
Comparing bond energy, C-Cl bond is longer and weaker.
Less energy is required to break C-Cl bond, and it should be more likely broken to form radicles •CClF2 and Cl• instead.
Hence statement 1 is false.
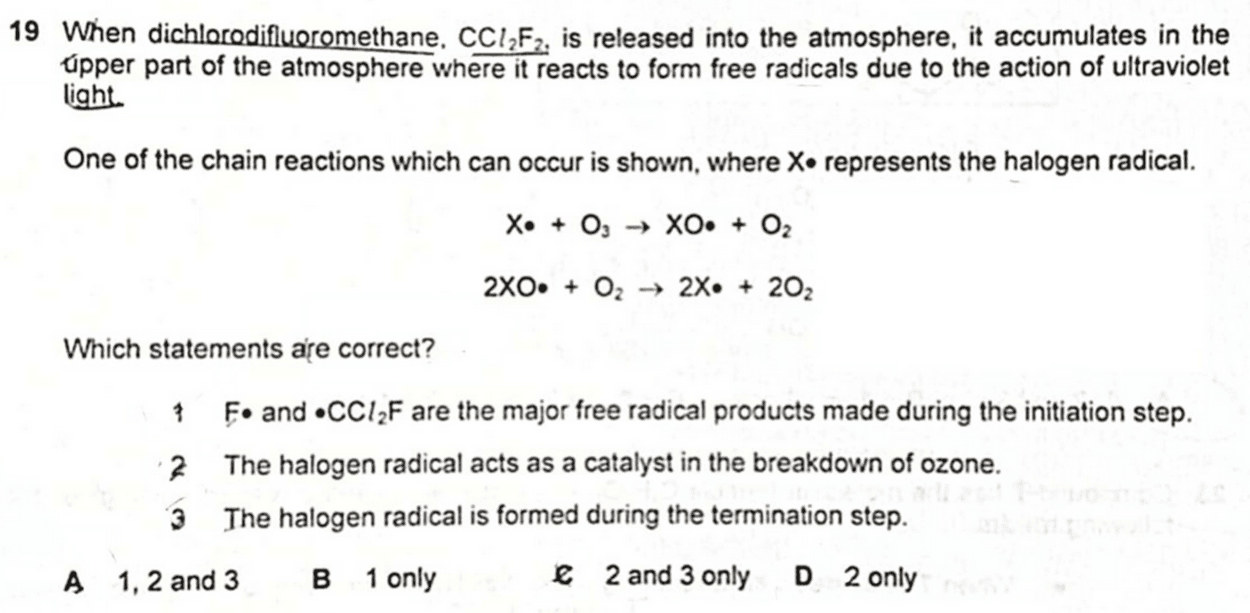
2. The halogen radical acts as a catalyst in the breakdown of ozone.
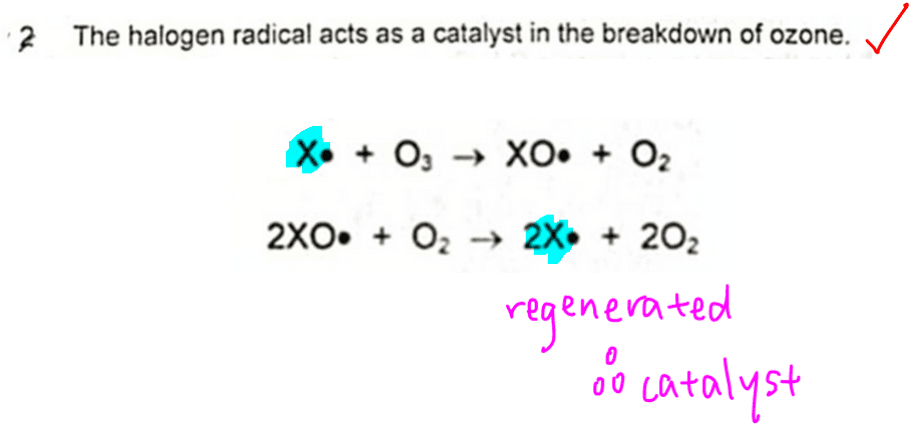
In the mechanism given, we notice radicle X• is used up in the first step and regenerated in the second step.
This suggests that X• is a catalyst.
Hence statement 2 is true.
3. The halogen radical is formed during the termination step.
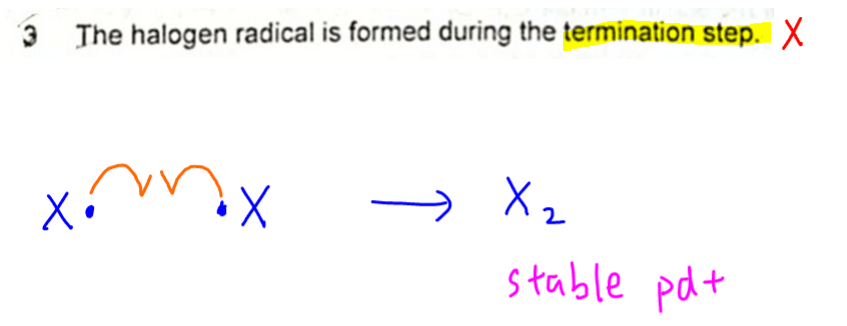
From the understanding of free radical substitution of alkanes, we know that termination step involves combining 2 radicals to form a stable compound.
Check out this video to learn more about free radical substitution of alkanes.
This means radicals are not formed during termination step.
Hence statement 3 is false.
We can now go through the options and determine the answer to this question is option D.

Topic: Alkanes & Halogenoalkanes, Organic Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
