2020 P1 Q21 - Comparing Reactivity of Chloro Compounds with AgNO3
Let Chemistry Guru, Singapore's top choice for JC Chemistry tuition, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 21.

We are asked to determine which compound is least reactive with a few drops of ethanolic silver nitrate.
Let us first compare the reactivity of the following chloro compounds in organic chemistry.
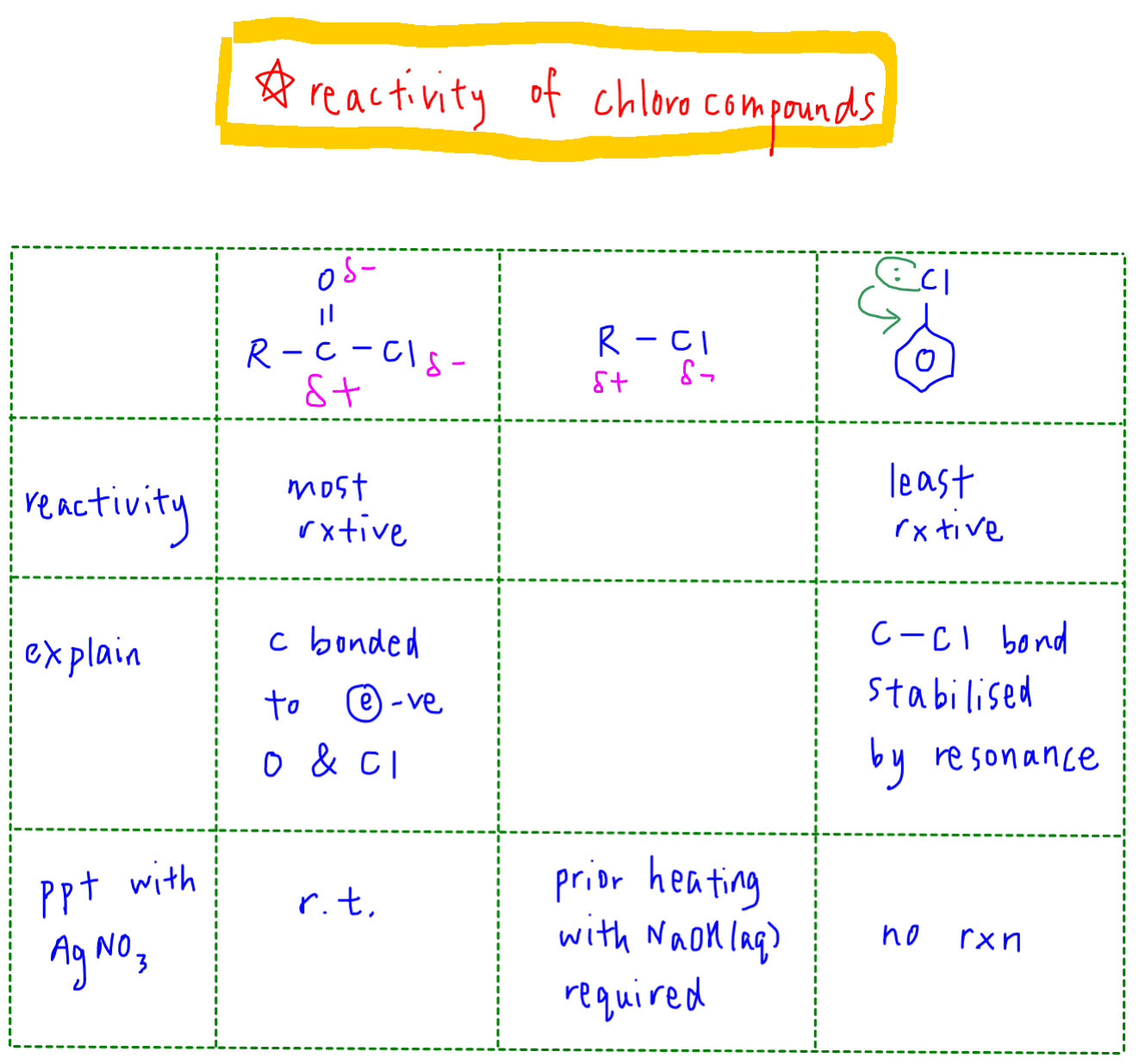
Acid chloride is the most reactive as the acid carbon is attached to 2 electronegative oxygen and chlorine atoms.
This makes the acyl carbon very positive and attractive to nucleophiles.
Acid chlorides will give immediate white ppt with AgNO3 without any heating required.
Next, halogenoalkane carbon is less positive since it is only attached to 1 electronegative chlorine.
Therefore it is less reactive than acid chlorides.
Prior heating with NaOH(aq) is required to break the C-Cl bond and release Cl- for precipitation with AgNO3.
Finally, chlorobenzene is the most stable as the C-Cl bond is resonance stabilised.
Lone pair from chlorine can interact with the delocalised pi system of benzene and the resonance stability is extended to C-Cl bond.
We can also say the C-Cl bond has partial double bond character.
Hence chlorobenzene does not undergo any nucleophilic substitution and no ppt will be formed with AgNO3.
We can now go through the options and determine the compound that is the least reactive should be option C - chlorobenzene.

Topic: Halogenoalkanes, Carboxylic Acid and Derivatives, Organic Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
