2020 P1 Q24 - Comparing Basicity of Amines
Let Chemistry Guru, Singapore's top choice for JC Chemistry tuition, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 24.
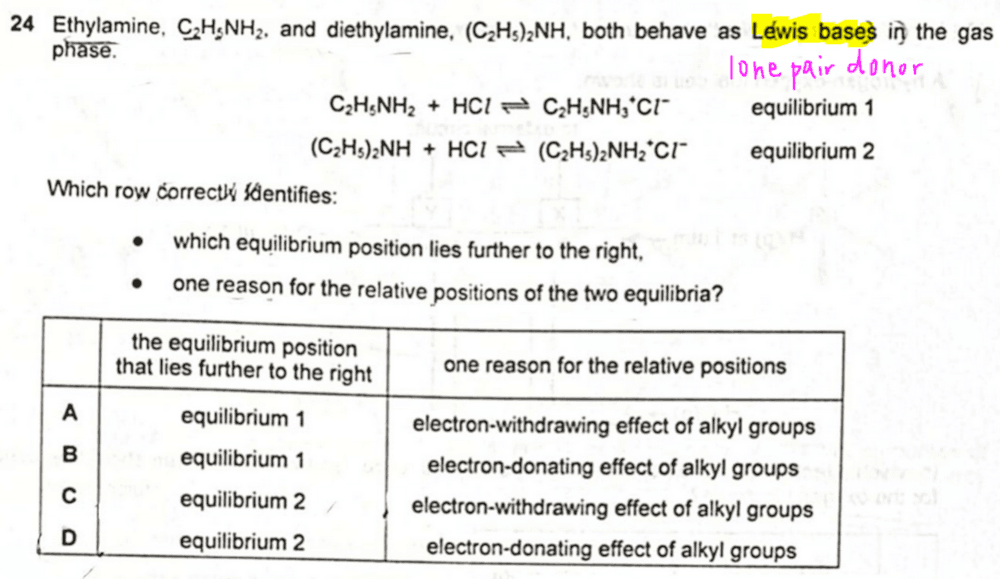
We need to compare and explain the equilibrium positions of the acid base reaction between ethylamine and diethylamine with HCl.
Let us start by comparing the basicity of ethylamine and diethylamine.
The basicity of nitrogen compounds is determined by the availability of lone pair on nitrogen.
The more available the lone pair on nitrogen, the stronger the lewis base.
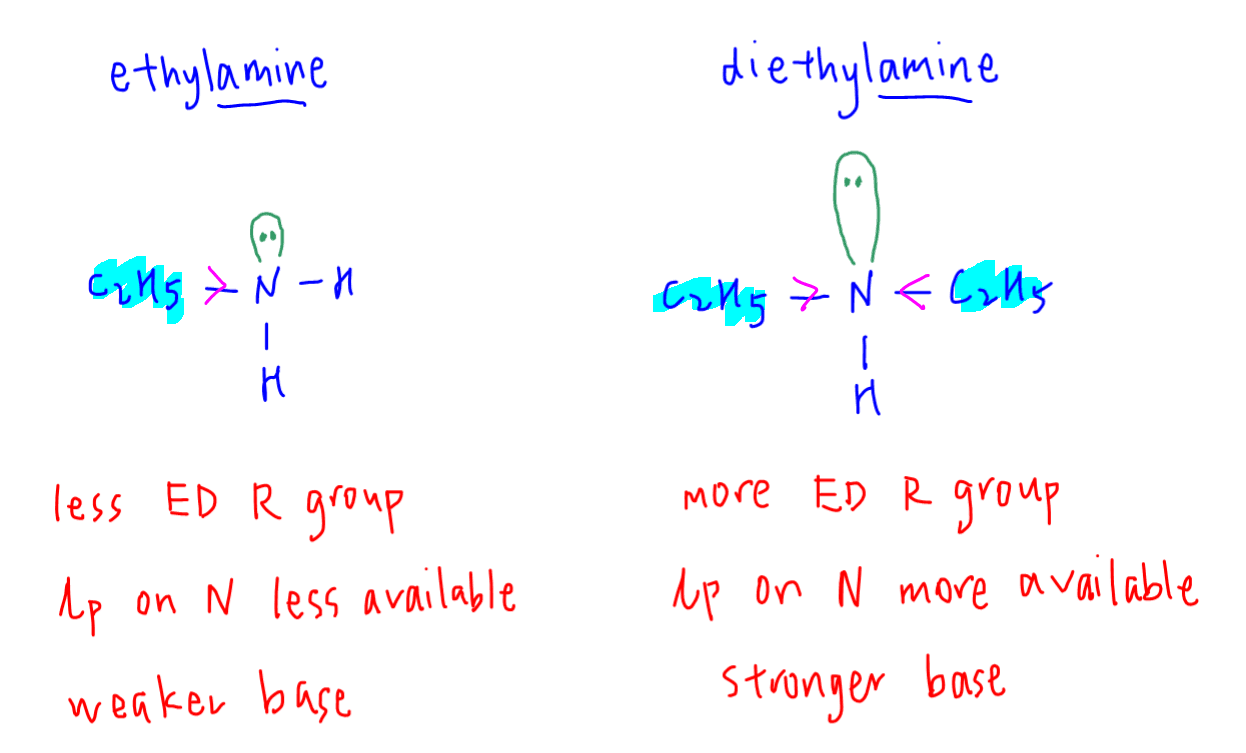
Ethylamine has only 1 electron donating alkyl group.
This makes the lone pair on nitrogen less available for donation.
Hence ethylamine should be a weaker base.
Diethylamine has 2 electron donating alkyl groups, which makes the lone pair on its nitrogen more available.
This means diethylamine is a stronger base than ethylamine.
In general the more substituted the amine, the stronger the base:
tertiary amine > secondary amine > primary amine > ammonia
Therefore we will expect the position of equilibrium for the second equilibrium to lie further to the right.
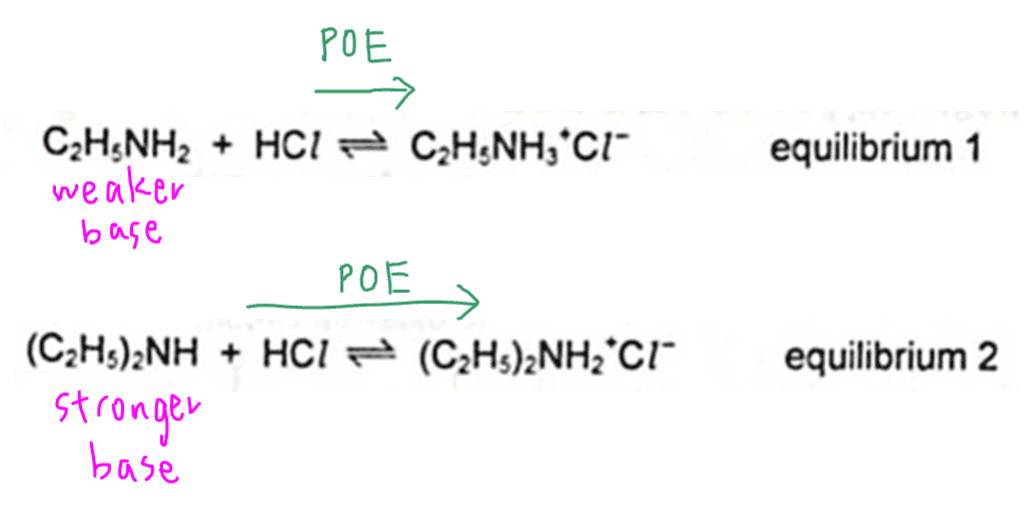
Finally we can compare the options and determine the answer to this question is option D.

Topic: Nitrogen Compounds, Organic Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's esteemed H2 Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
