2020 P1 Q3 - Determine Charge from Proton, Neutron and Electron Number
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 3.

We are required to determine this unknown ion by deducing the proton, neutron and electron number.
We know that atomic or proton number is from 84 to 87. Let's find out what these elements are.
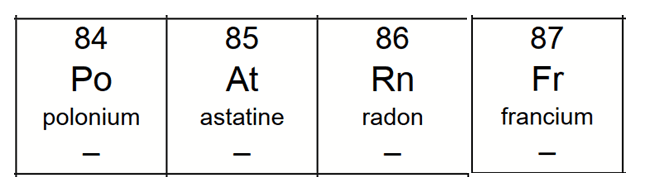
Now we can figure out the proton number, neutron number, electron number and charge of each element:
- proton number is the same as atomic number
- neutron number is nucleon number 217 minus proton number
- electron number is neutron number minus 50
- charge of ion is proton number less electron number

From the calculations we will have the following ions: Po+, At3+, Rn5+, Fr7+.
We can now compare with the options and see which is consistent.

Option B with At3+ should be the correct answer.
Topic: Atomic Structure, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
