2020 P1 Q30 - Deducing Density and Melting Point of Vanadium
Let Chemistry Guru, Singapore's top choice for JC Chemistry tuition, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 30.
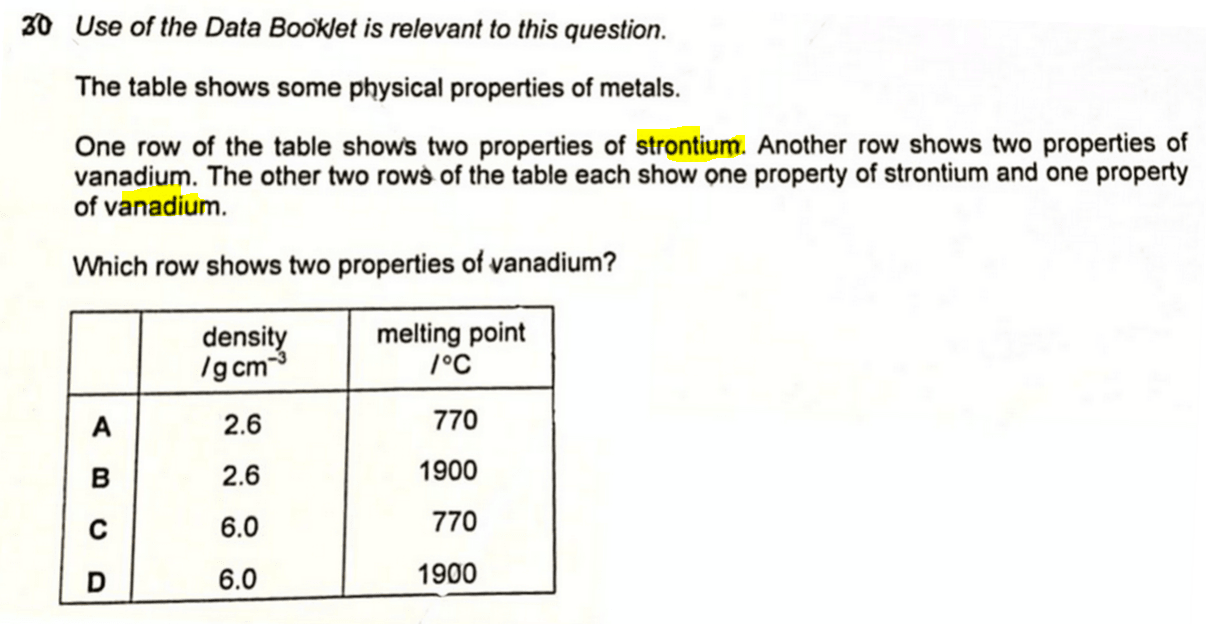
We are required to determine which row shows the density and melting point of vanadium.
Let's locate these 2 metals in the Periodic Table and consider their atomic radii too.
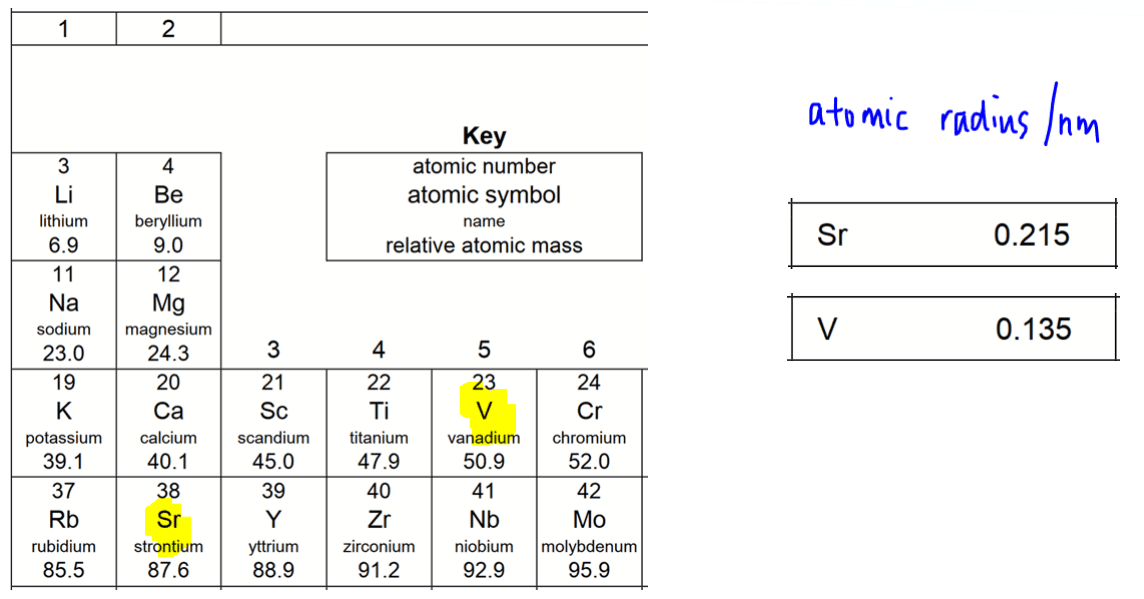
Strontium is a Group 2 metal in Period 5, while vanadium is a transition metal in Period 4.
Since they are in different periods we have to be careful in comparing their properties.
In syllabus the default comparison is between group 2 calcium and transition metals in Period 4.

Transition metal atoms have bigger mass number and smaller size hence more atoms can be packed per unit volume.
This means transition metals should have higher density as compared to group 2 metals like calcium.
Since strontium is in a different period, we want to make sure this comparison is still valid.
Strontium mass number 87.6, atomic radius 0.215 nm
Vanadium mass number 50.9, atomic radius 0.135 nm
Notice vanadium has a smaller mass number and smaller size than strontium.
This means we cannot use the comparison between calcium and transition metals in period 4 to deduce their densities directly.
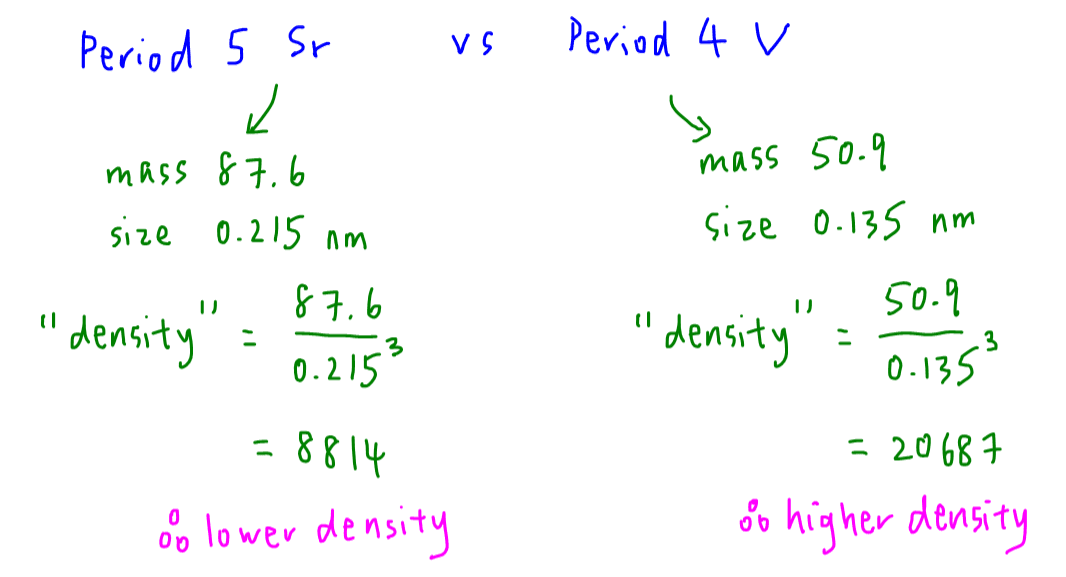
Hence let's estimate density via this equation:
density = mass / volume = mass / radius3
Note the accuracy of the equation is not important as long as we apply the same equation to both strontium and vanadium for fair comparison.
density of strontium = 8814
density of vanadium = 20687
Hence vanadium should have a higher density than strontium.
Next let's compare their melting points which is much more straightforward.

Transition metals can delocalise their 4s and 3d electrons hence more positively charged cations will attract a bigger sea of delocalised electrons.
This means transition metals will have stronger metallic bonds and higher melting points compared to group 2 metals.
Hence vanadium should have a higher melting point than strontium.
We can now look through the options and determine the answer is D.

Topic: Transition Elements, Inorganic Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's esteemed H2 Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
