2020 P1 Q4 - Compare Boiling Points of C5H12 Isomers
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 4.
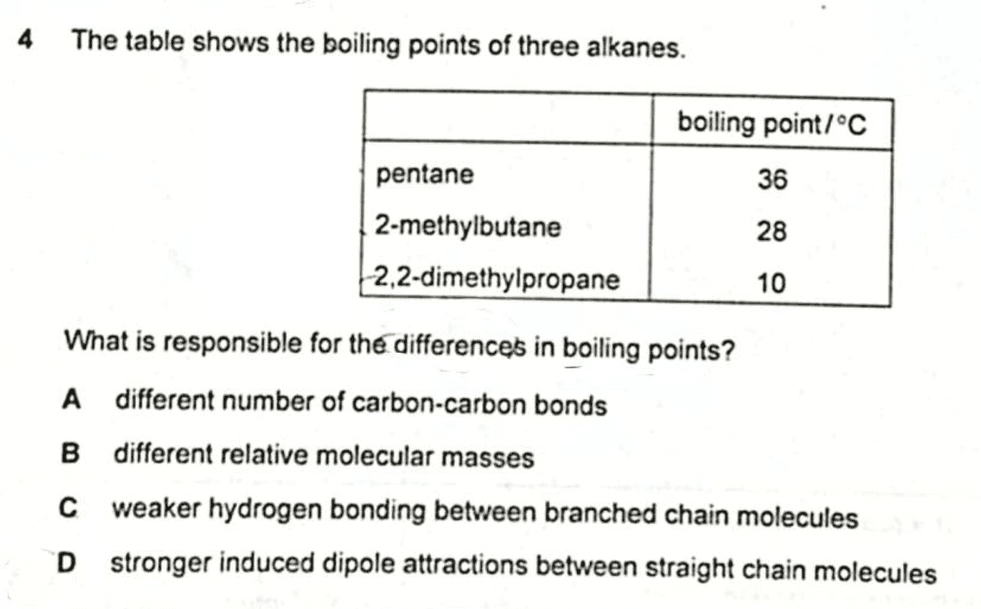
We are required to explain the boiling point differences for pentane, 2-methylbutane and 2,2-dimethylpropane.
Since they are non-polar alkanes, their dominant intermolecular forces will be instantaneous dipole - induced dipole attractions.
Let's recap the factors affecting id - id attractions.
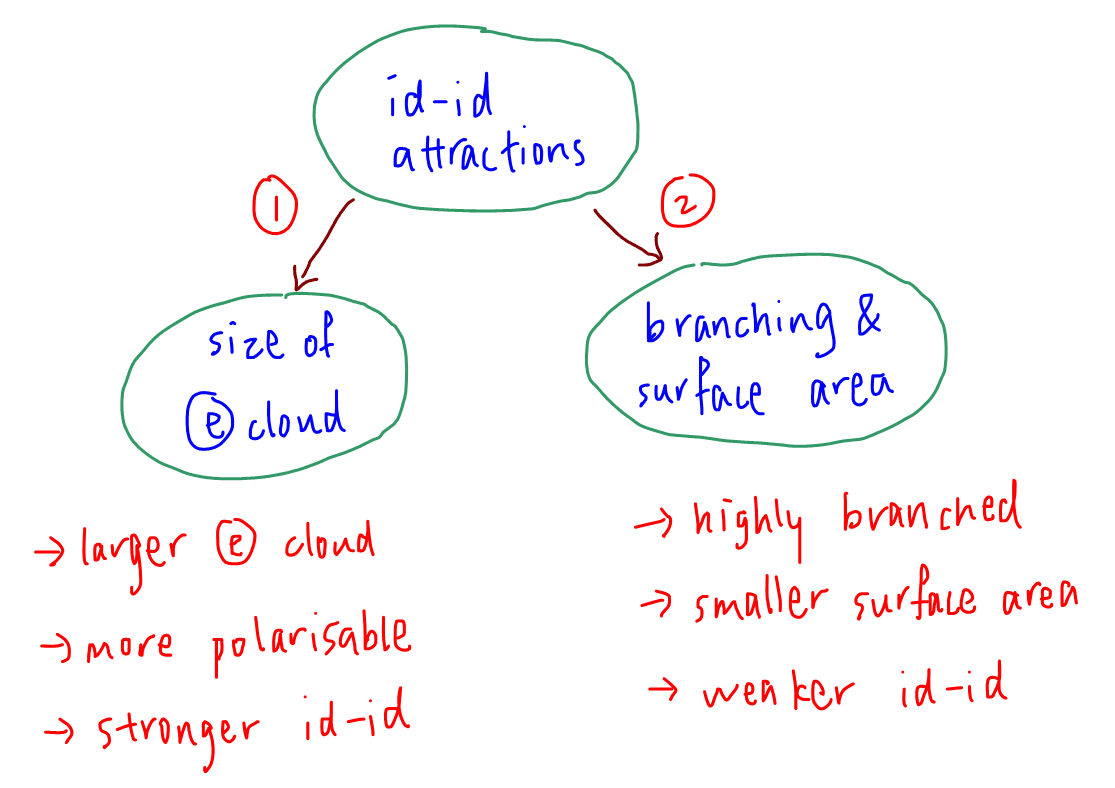
1. Size of Electron Cloud
The molecule with a larger molar mass will have a bigger and more polarisable electron cloud.
Id - id attraction between larger molecules will be stronger and boiling point will be higher.
When comparing isomers with the same electron cloud size, we have to look at the second factor instead.
2. Branching and Surface Area for Molecular Interaction
For comparing isomers, the more highly branched molecule will be more spherical and have a smaller surface area to interact with neighboring molecules.
Hence id - id attraction between the more spherical molecules is weaker and boiling point will be lower.
Since pentane, 2-methylbutane and 2,2-dimethylpropane have the same molecular formula C5H12, they are chain isomers of each other.
So we will use the second factor involving surface area to compare their boiling points.
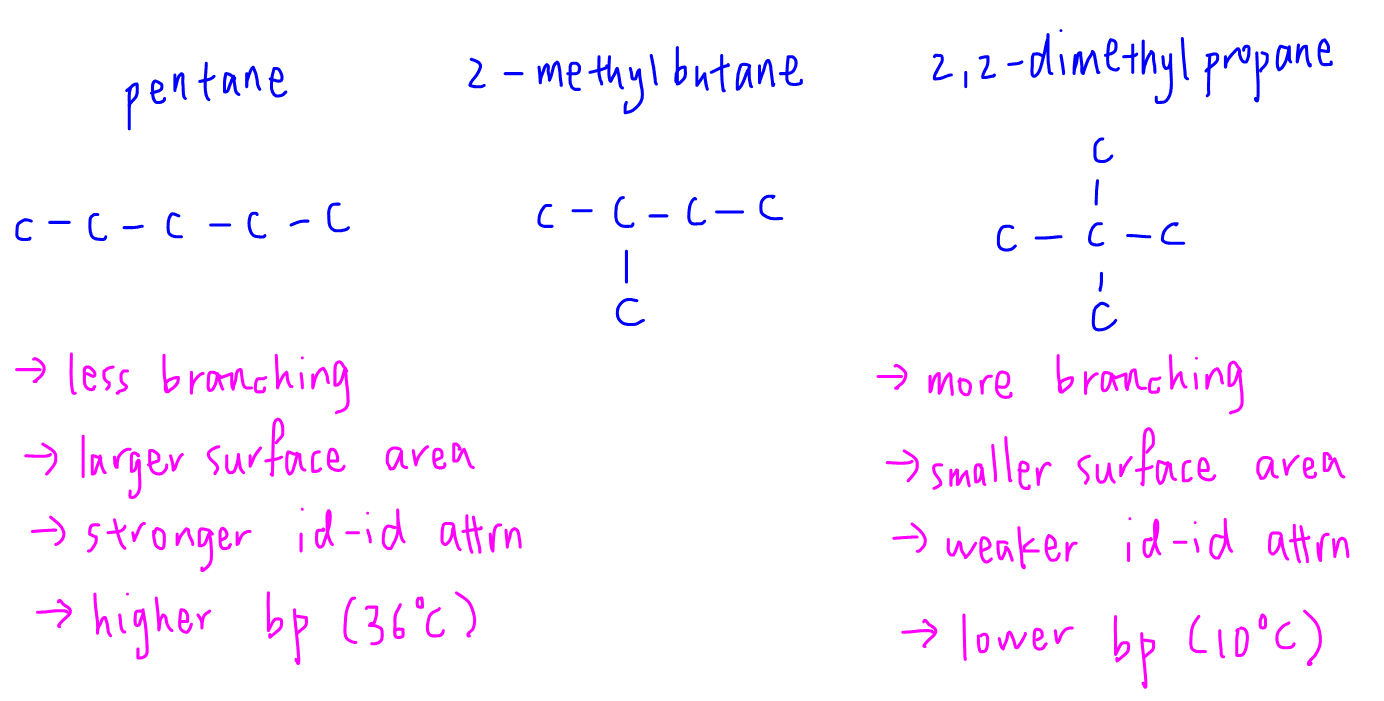
Pentane is unbranched hence has the largest surface area for molecular interaction.
Id - id attraction between pentane is the strongest and boiling point will be the highest.
2,2-dimethylpropane, on the other hand, is the most highly branched.
It is the most spherical with the smallest surface area for interaction between molecules.
Hence id - id attraction between 2,2-dimethylpropane molecules is the weakest and boiling point is the lowest.
Let's look at the options and determine our answer.

Option A is not relevant since number of carbon-carbon bonds does not affect physical properties such as boiling point.
Option B is not correct as the 3 alkanes in this question have the same molar mass and electron cloud size.
Option C is not valid as non-polar molecules cannot form hydrogen bonds between molecules.
Option D is our best answer based on what we have discussed earlier.
Topic: Intermolecular Forces, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
