2020 P1 Q6 - Compare PV against V Graph of Equal Masses of Gases
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 6.
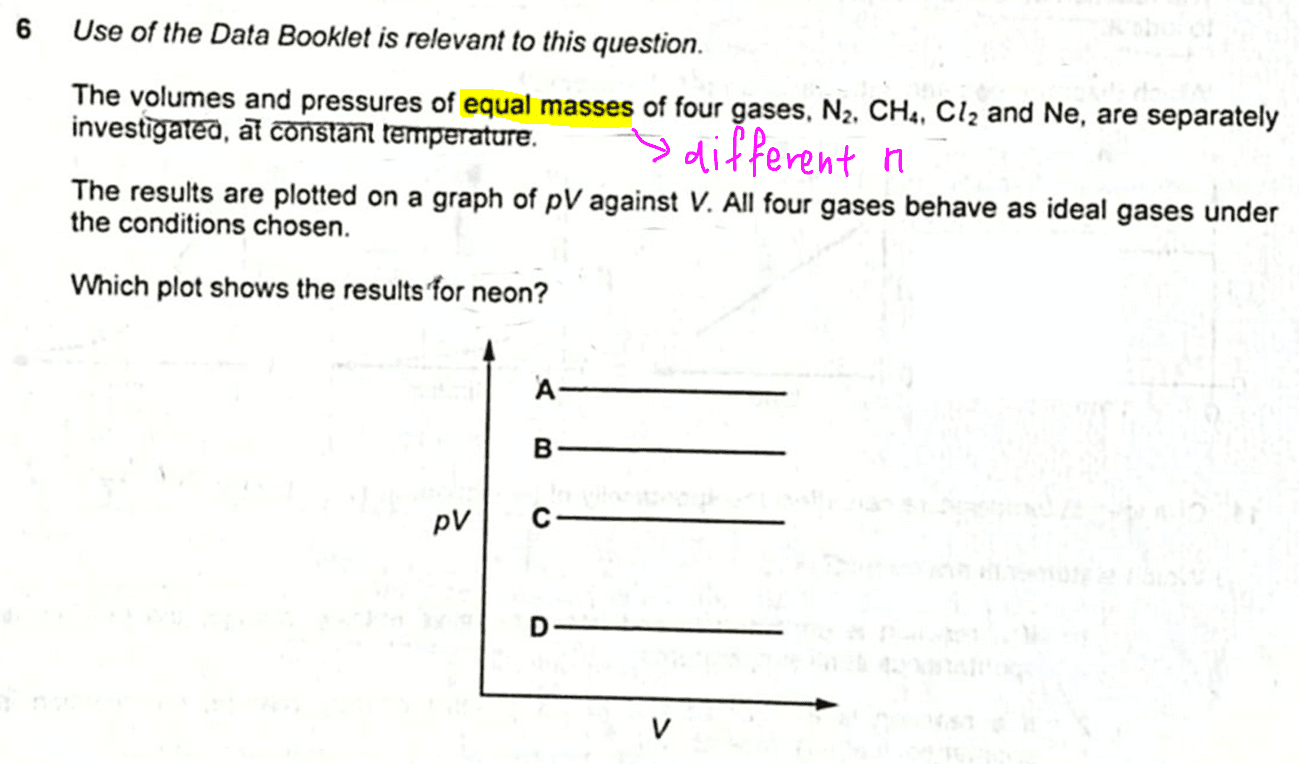
We are required to determine which graph shows the results for neon.
Let us first consider the shape of PV against V graph for an ideal gas.

From Ideal Gas Equation
PV = nRT
Gas constant R and temperature T are constants and for each gas, number of moles n will also be constant.
This means nRT is a constant and we can write the Ideal Gas Equation as
PV = nRT = k
The graph of PV against V will be a horizontal line, and the y-intercept will be k.
Next let us consider different gases of equal mass.
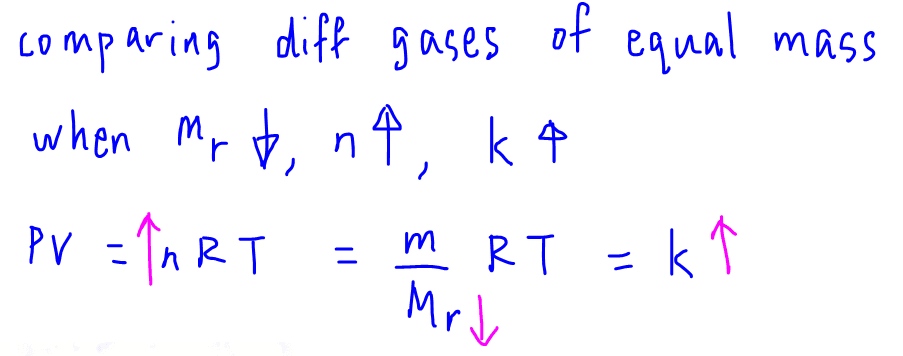
A gas with a smaller molar mass will have a greater number of moles.
Since y-intercept k = nRT, larger n means k will also be larger.
This means a gas with smaller molar mass will have larger y-intercept k.
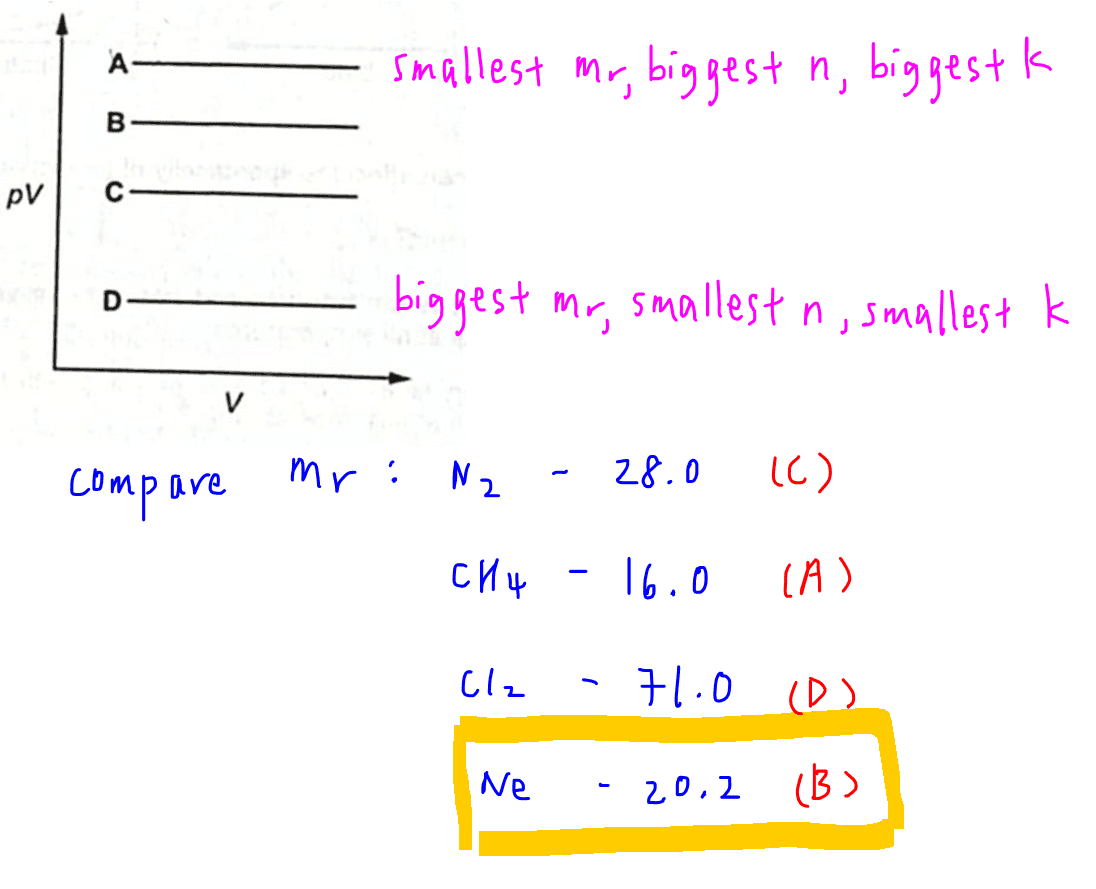
We can now compare the molar mass of the gases given and determine which graph is neon.
CH4 has the smallest molar mass of 16.0 hence largest n and k that corresponds to graph A.
Ne has the next smallest molar mass of 20.2 hence second largest n and k that corresponds to graph B.
Cl2 has the largest molar mass of 71.0 hence its moles and k will be the smallest and that would correspond to graph D.
Therefore the answer to this question will be option B.
Topic: Gaseous State, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
