2020 P1 Q7 - Determine Partial Pressure of HI in Equilibrium Mixture
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 7.

We need to find the partial pressure of HI at equilibrium.
First let us fill up the ICE table that represents the initial, change and equilibrium moles of reactants and products.
Check out the following video to learn how to use the ICE table in Chemical Equilbria questions.
Notice we are given 0.040 moles of HI which is the product.
This means the reverse reaction will take place and HI will be used up while H2 and I2 are formed.
We can let the change in H2 be x, and fill up the following ICE table accordingly.

Notice we have an additional row calculating the partial pressure of each species at equilibrium.
Partial pressure of a gas is its mole fraction multiply by the total pressure.
This is required since the equilibrium constant given, Kp, is in terms of partial pressure.
Once we have the partial pressures at equilibrium we can substitute these values into the equilibrium constant expression and solve for x.
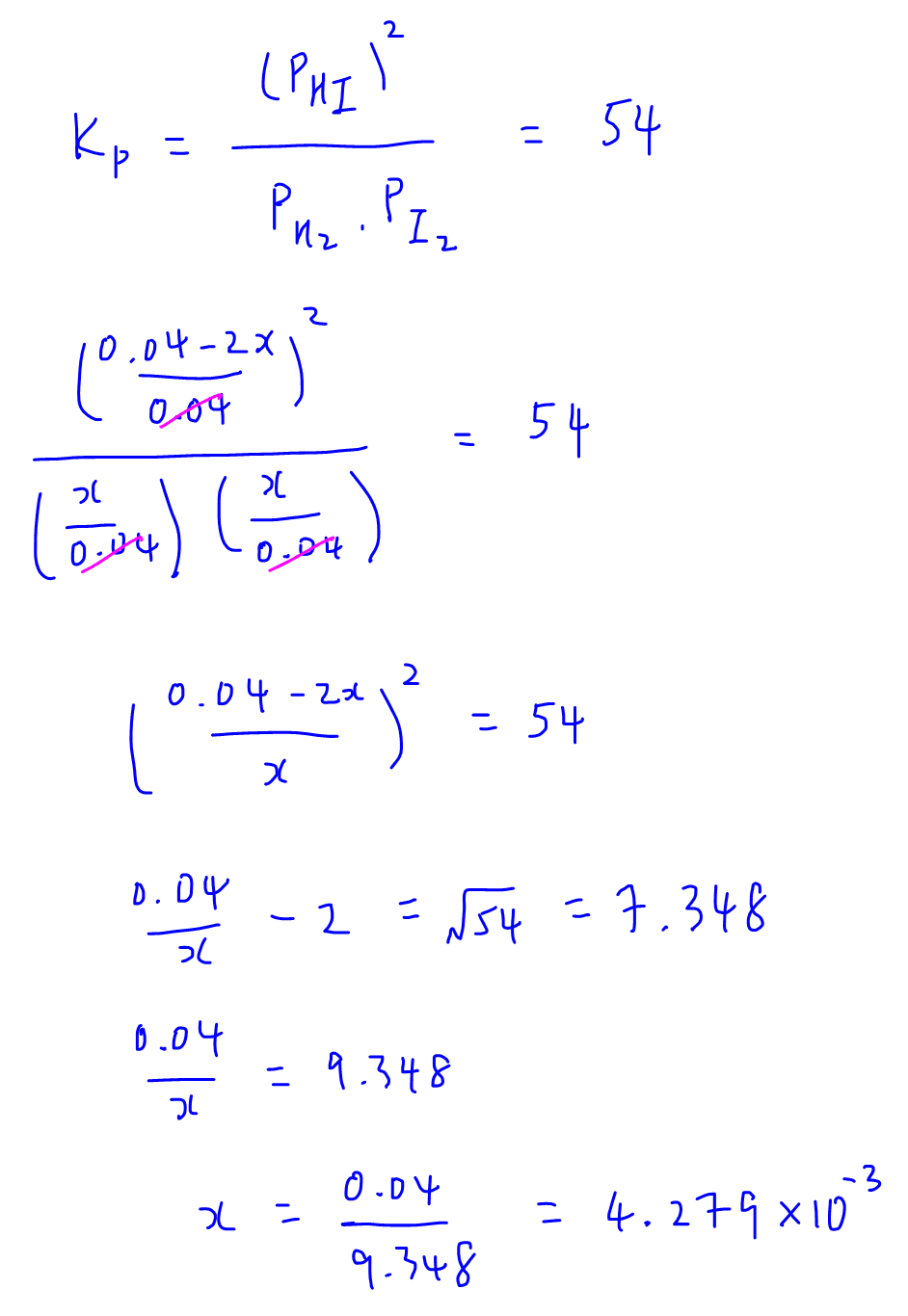
Remember x is not the final answer so we have to use x to solve for partial pressure of HI at equilibrium.

Therefore the answer to this question will be option B.
Topic: Chemical Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
