2020 P1 Q8 - Thermal Decomposition of Group 2 Carbonates
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 8.

We want to explain why MgCO3 decomposes at a lower temperature than CaCO3.
The concept tested in this question is on thermal decomposition of Group 2 metal carbonates.
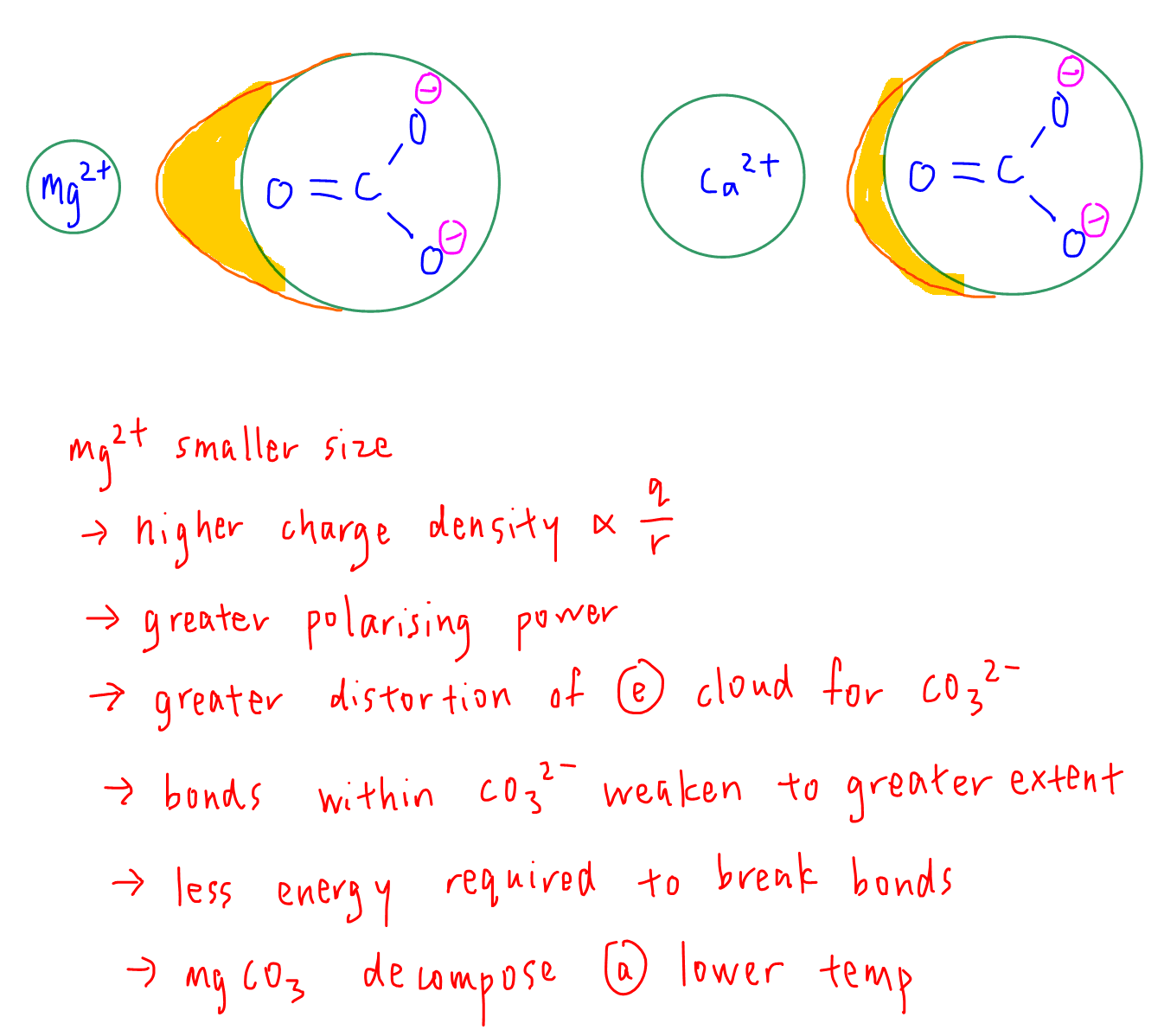
Mg2+ is smaller than Ca2+, hence Mg2+ has a higher charge density and greater polarising power.
This causes a greater distortion of electron cloud for CO32- which weakens the bonds within the anion to a greater extent.
Less energy is required to break the covalent bonds in CO32- hence MgCO3 decomposes at a lower temperature.
Let's go through the options and see which is the best answer.

Option A is relevant as the charge density of Mg2+ is higher which makes it more polarising than Ca2+ as explained above.
Option B is not relevant as formation of metal oxide from its constituent elements (metal M and oxygen O2) is not related to metal carbonate at all.
Option C is not relevant as lattice energy is related to energy released when the ionic compound is formed from its constituent ions.
This involves the attraction between the M2+ and CO32- ions and is not related to breaking of covalent bonds within the carbonate anion.
Option D is not relevant as melting is a physical process so is not related to the decomposition of metal carbonate which is a chemical reaction.
Hence the answer to this question is option A.
Topic: Group 2 and 17 Elements, Inorganic Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
