2020 P1 Q14 - Deduce Enthalpy Change by Comparing Kc at Different Temperatures
Let Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre, guide you through 2020 A Levels H2 Chemistry Paper 1 Question 14.
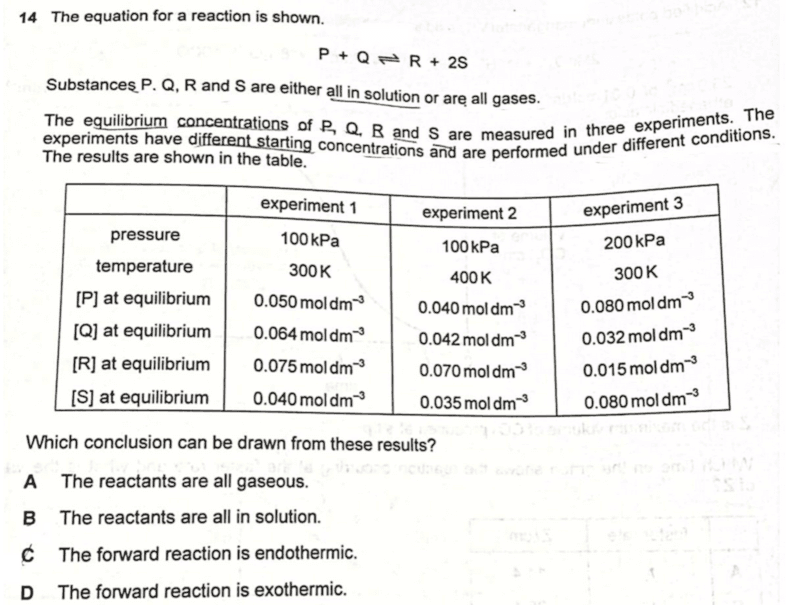
We are asked to determine which of the conclusions can be drawn from the results.
Let's start by writing down the equilibrium constant for this reaction.

Since the equilibrium concentrations of reactants and products are given, we can substitute these values to calculate Kc for each experiment.

Notice Kc for experiments 1 and 3 are the same since temperature is the same at 300K.
We know that Kc will remain constant when temperature is unchanged.
Other factors like concentration of reactants or products, pressure or presence of catalyst will not affect Kc.
For experiment 2 at a higher temperature of 400K, Kc is larger.
This means that Kc increases at a higher temperature, and we can deduce the enthalpy change for this reaction.
First let us consider what an increase in Kc mean to the position of equilibrium.
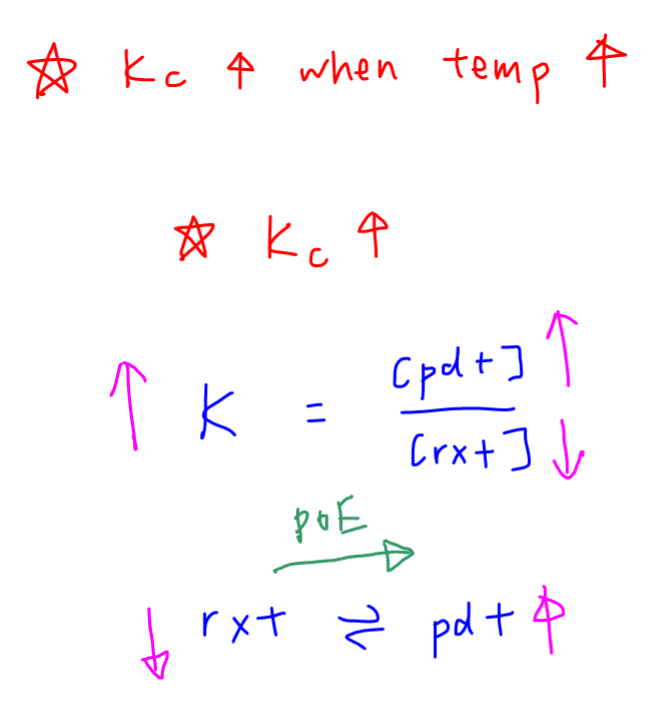
A larger Kc will mean a higher product concentration and lower reactant concentration.
Since more products are formed and less reactants are left, this must mean the position of equilibrium is shifted towards the right hand side.
This also means the forward reaction is favoured.
Conclusion 1 - When temperature increases, the forward reaction is favoured.
Next we can deduce whether an increase in temperature would favour exothermic or endothermic reaction.
According to Le Chatelier's Principle, the system will favour the endothermic reaction when temperature increases to absorb excess heat.
Conclusion 2 - When temperature increases, the endothermic reaction is favoured.
Check out this video lesson for a more detailed discussion about Le Chatelier's Principle and Position of Equilibrium.
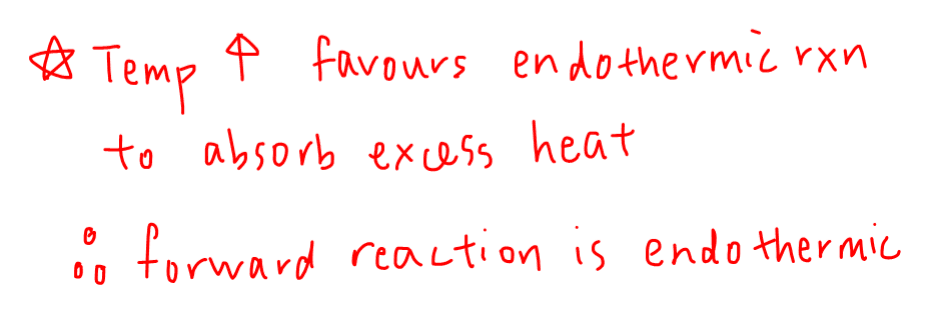
We can now put both conclusions together to deduce that the forward reaction is endothermic.

Therefore the answer to this question will be option C.
Topic: Chemical Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2020 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
