2021 P1 Q10 - Determining Greatest Number of Molecules
Let Chemistry Guru, Singapore's top choice for JC Chemistry tuition, guide you through 2021 A Levels H2 Chemistry Paper 1 Question 10.
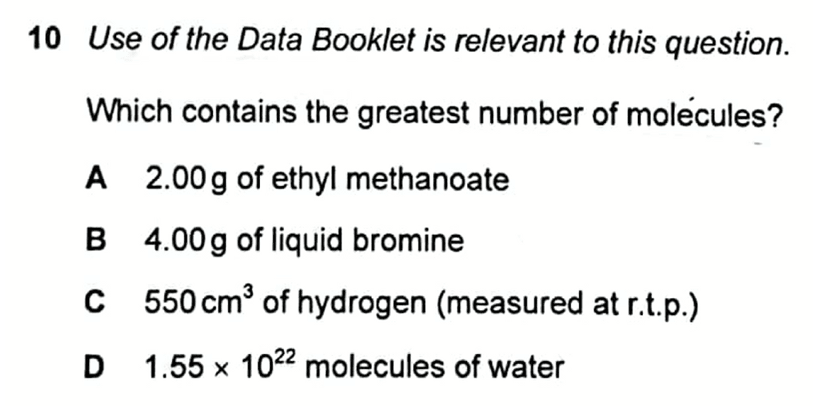
We are required to determine which option contains the greatest number of molecules.

Since number of molecules is directly related to number of moles, we can just calculate the moles for each option instead.
Option A - 2.00g of ethyl methanoate

The molar mass for ethyl methanoate, C3H6O2, is 74.0.
Number of moles will be mass over molar mass = 2.70 x 10-2
Option B - 4.00g of liquid bromine
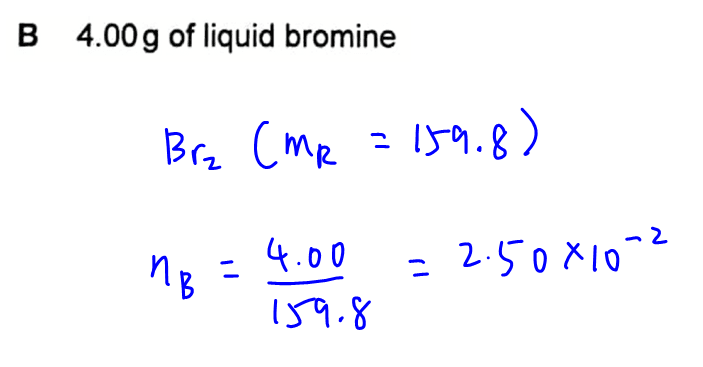
Molar mass for bromine, Br2, is 159.8.
Number of moles will be mass over molar mass = 2.50 x 10-2
Option C - 550 cm3 of hydrogen measured at rtp

Molar volume of a gas at room temperature and pressure is 24.0 dm3.
Number of moles will be volume in dm3 over molar volume = 2.29 x 10-2
Option D - 1.55 x 1022 molecules of water
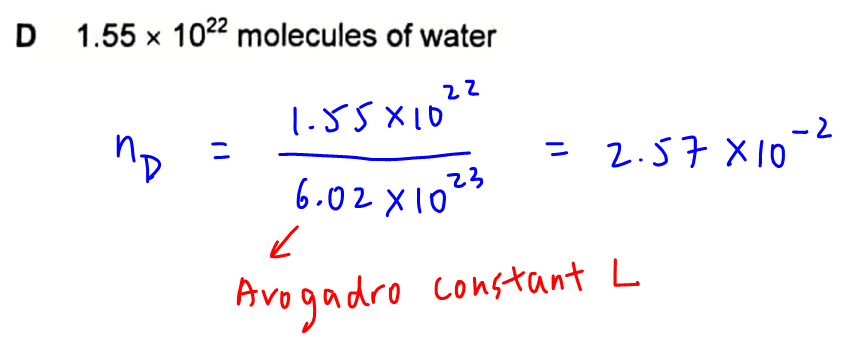
Number of moles will be number of molecules over Avogadro constant L = 2.57 x 10-2
We can now compare all the options to determine which has the greatest moles (and greatest number of molecules).
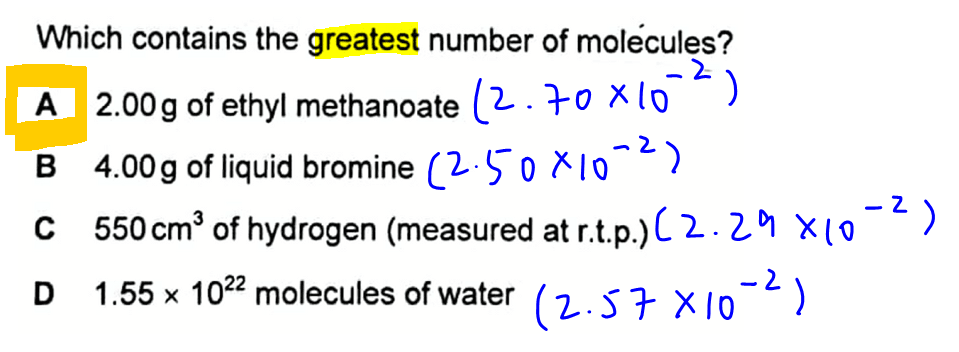
The answer to this question is option A (2.70 x 10-2).
This question is not difficult but can be pretty tedious.
Hence we have to be familiar with our mole concept calculations to attempt this question efficiently.
Topic: Mole Concept, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2021 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's esteemed H2 Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
