2021 P1 Q11 - Calculate Enthalpy Change of Neutralisation
Let Chemistry Guru, Singapore's top choice for JC Chemistry tuition, guide you through 2021 A Levels H2 Chemistry Paper 1 Question 11.
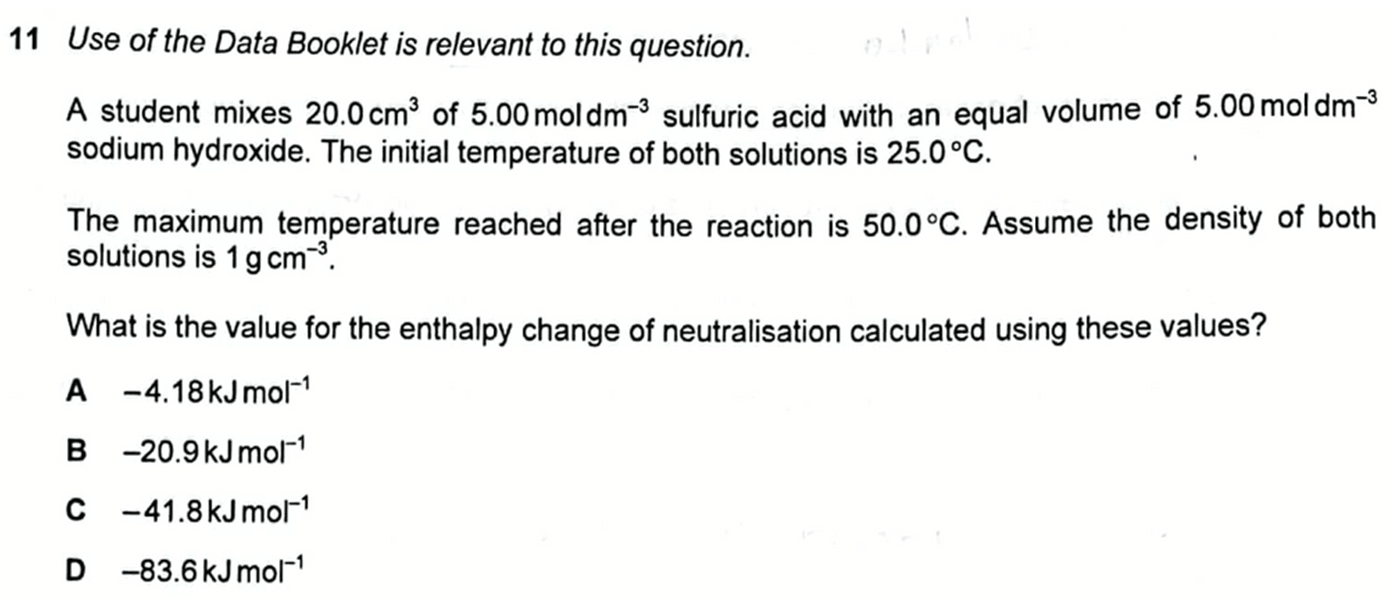
We are required to calculate the enthalpy change of neutralisation for the mentioned experiment.
Using a diagram is useful to help us visualise the process.
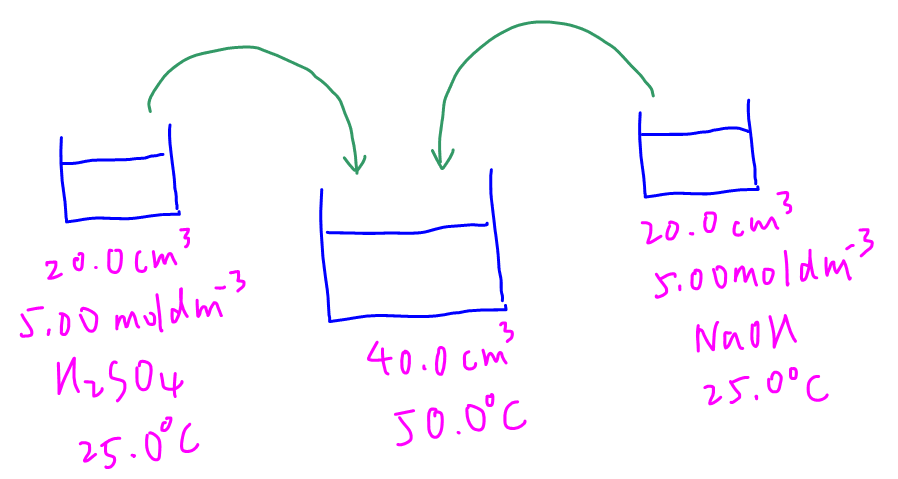
We can use the following steps to calculate enthalpy change for calorimetry questions.
1. Determine heat released by neutralisation
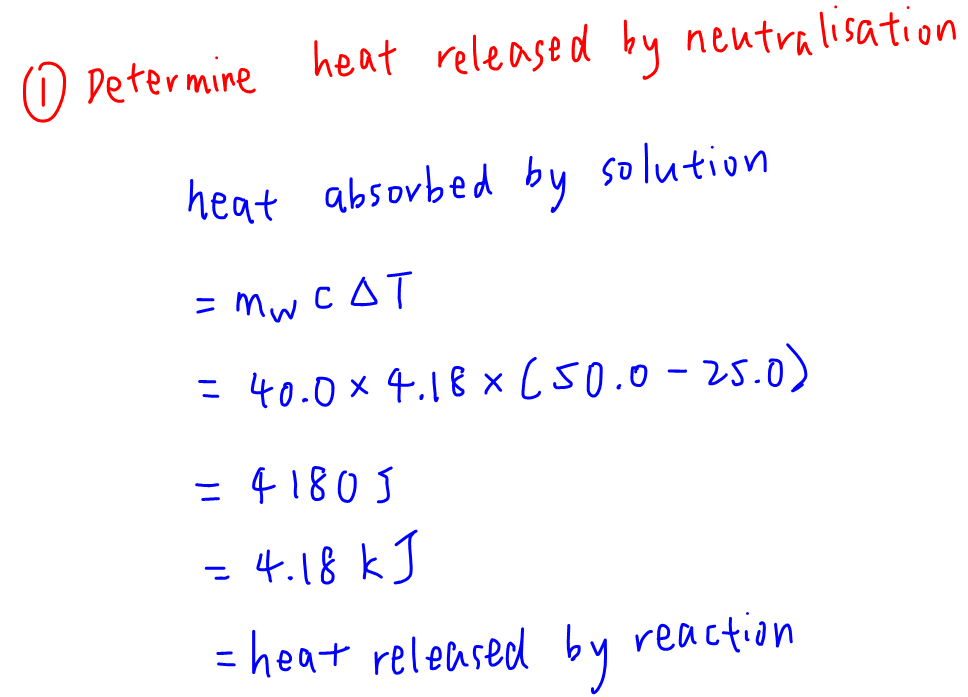
Heat absorbed by water is given by mass of water x specific heat capacity of water x change in temperature of water.
heat absorbed by solution = mw.c.ΔT
We can calculate heat absorbed to be 4.18 kJ.
Assuming no heat loss to surrounding, heat released by reaction will be the same value 4.18 kJ.
2. Determine limiting reagent

We can calculate moles of reactants H2SO4 and NaOH to be 0.1 mol.
Hence NaOH is limiting.
Since enthalpy change of neutralisation is with respect to per mole of water, we need to calculate moles of water formed from the limiting reagent.
Hence moles of water formed = moles of NaOH = 0.1 mol
3. Calculate enthalpy change of neutralisation
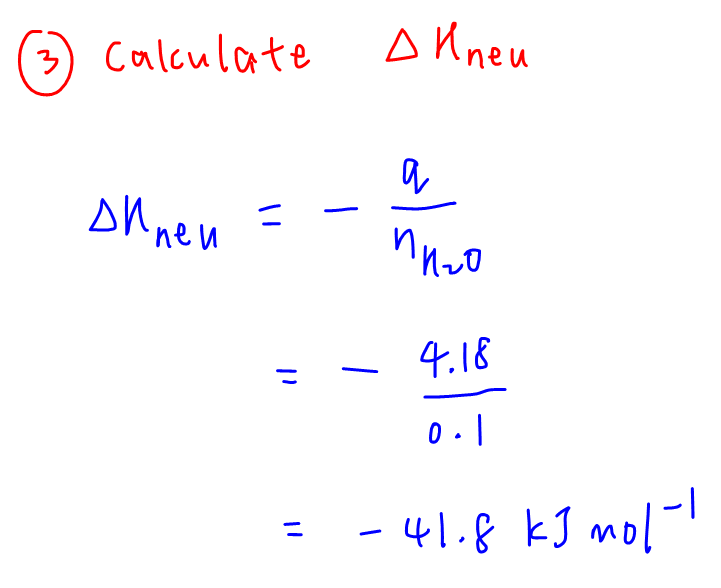
Enthalpy change is - q / moles of water which works out to be -41.8 kJ mol-1.
Note we put a negative sign for enthalpy change of neutralisation since the reaction is exothermic.
Finally we can compare the options to determine the answer to this question is option C.

Topic: Energetics, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2021 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's esteemed H2 Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
