2021 P1 Q12 - Deduce Change in Rate of Forward Reaction when POE shifts
Let Chemistry Guru, Singapore's top choice for JC Chemistry tuition, guide you through 2021 A Levels H2 Chemistry Paper 1 Question 12.

We need to deduce the change in the rate of forward reaction during this pressure change.
Let's break this down into 2 parts.
1. Deduce POE and rate of forward reaction at time t
At time t, the partial pressure of CO2 is lowered.
According to Le Chatelier's Principle, position of equilibrium will shift to the left to form more reactant CO2 and increase its partial pressure to counter the change imposed.
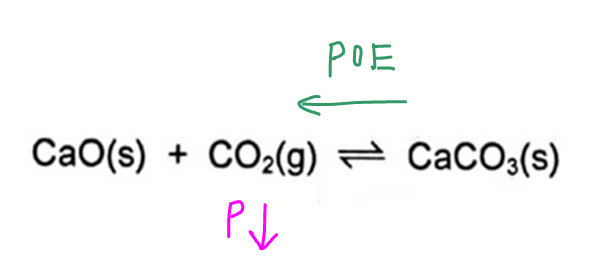
The reverse reaction is favoured and forward reaction is disfavoured.
Hence rate of forward reaction will decrease.
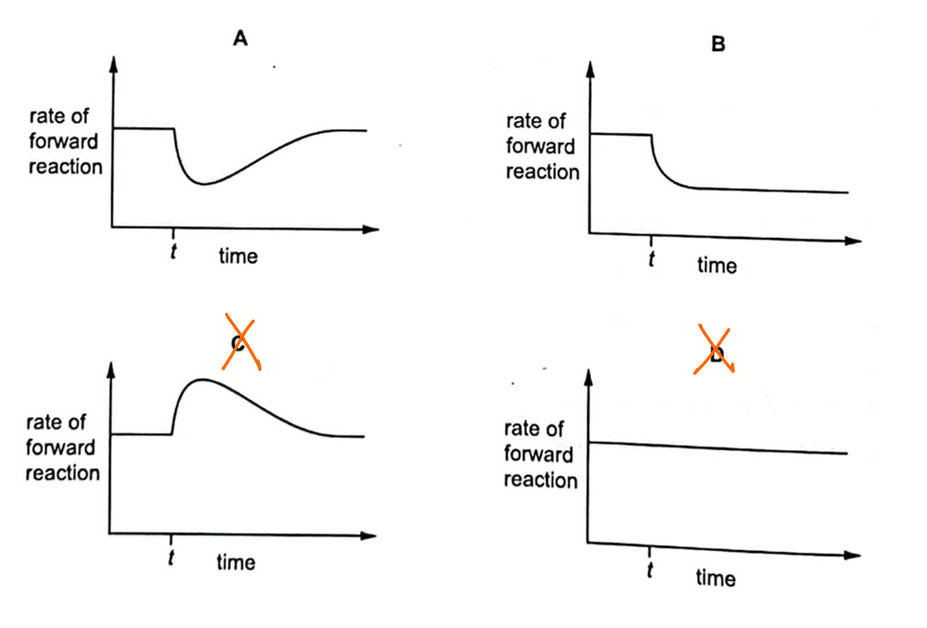
We can eliminate options C and D since there is no decrease in rate of forward reaction.
Check out the following video lesson to learn more about Le Chatelier's Principle and other changes that affect the position of equilibrium.
2. Deduce rate after return to atmospheric pressure
The pressure returns to original atmospheric pressure at constant temperature.
This means that the final state will have the same temperature and pressure as the original state.
We would then expect the rate of forward reaction to return to its original value also.
Therefore option A will be the better answer to this question.

If the pressure stays low after time t, then option B will be the better answer.
Topic: Chemical Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2021 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's esteemed H2 Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
