2021 P1 Q25 - Redox Reaction between Chromite and Oxygen
Let Chemistry Guru, Singapore's top choice for JC Chemistry tuition, guide you through 2021 A Levels H2 Chemistry Paper 1 Question 25.

We need to determine the moles of oxygen molecules required to react with one mole of chromite ions.
First we need to determine which species is oxidised and reduced in this redox reaction.
There is an increase in oxidation state of chromium from +3 in chromite Cr2O42- to +6 in chromate CrO42-.
Hence chromite is oxidised while oxygen is reduced.
Since the reaction is done in molten sodium hydroxide, oxygen should be reduced to hydroxide ions.
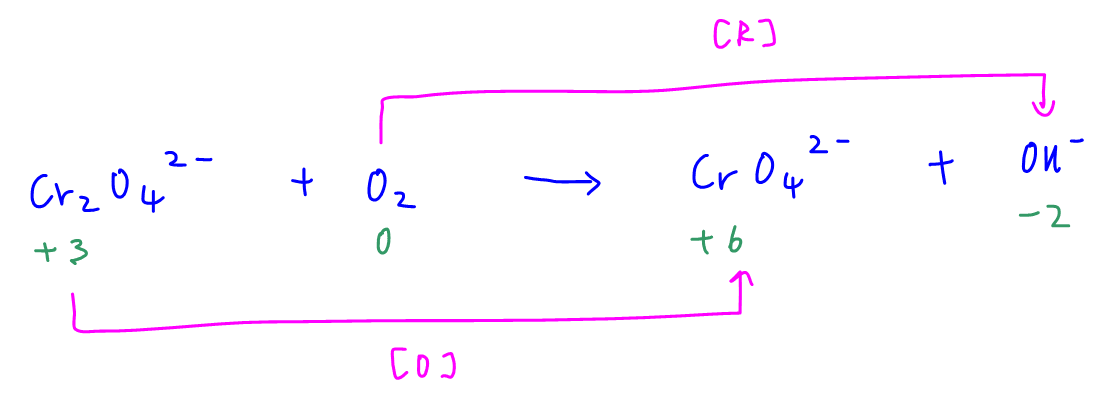
There are 2 ways to determine the mole ratio between oxygen molecule and chromite.
1. Balance Redox Equation
We can balance this redox reaction using half equation method to determine the mole ratio.
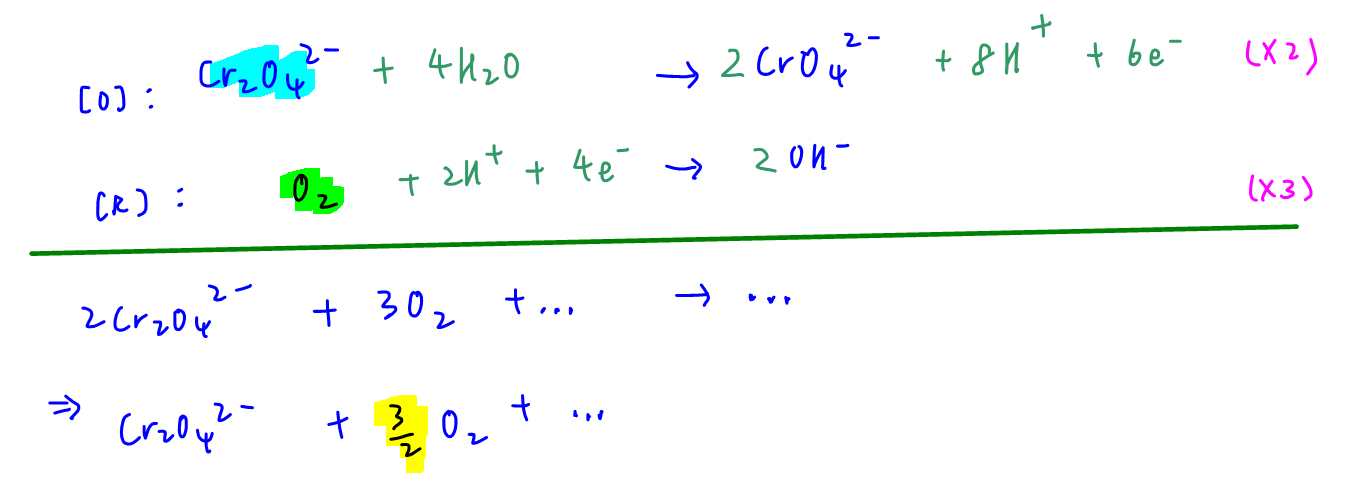
Check out this video to learn how to balance half equation for redox reactions.
Since we only need the mole ratio of the reactants, we can ignore the moles of other species involved such as the products CrO42- and OH-.
From the balanced equation we can determine mole ratio of O2 to Cr2O42- is 3 to 2.
Hence answer to this question is option B.

2. Compare Oxidation State
Oxidation:
There is an increase in oxidation state of chromium from +3 in chromite Cr2O42- to +6 in chromate CrO42-.
Each Cr oxidation state increases by 3 units, ie loses 3 electrons.
Hence each Cr2O42- loses 6 electrons.
Reduction:
There is a decrease in oxidation state of oxygen from 0 in oxygen molecule O2 to -2 in hydroxide OH-.
Each O oxidation state decreases by 2 units, ie gains 2 electrons.
Hence each O2 gains 4 electrons.
Putting it together, since moles of electrons lost by 1 mole of chromite is equal to moles of electrons gained by x moles of oxygen:
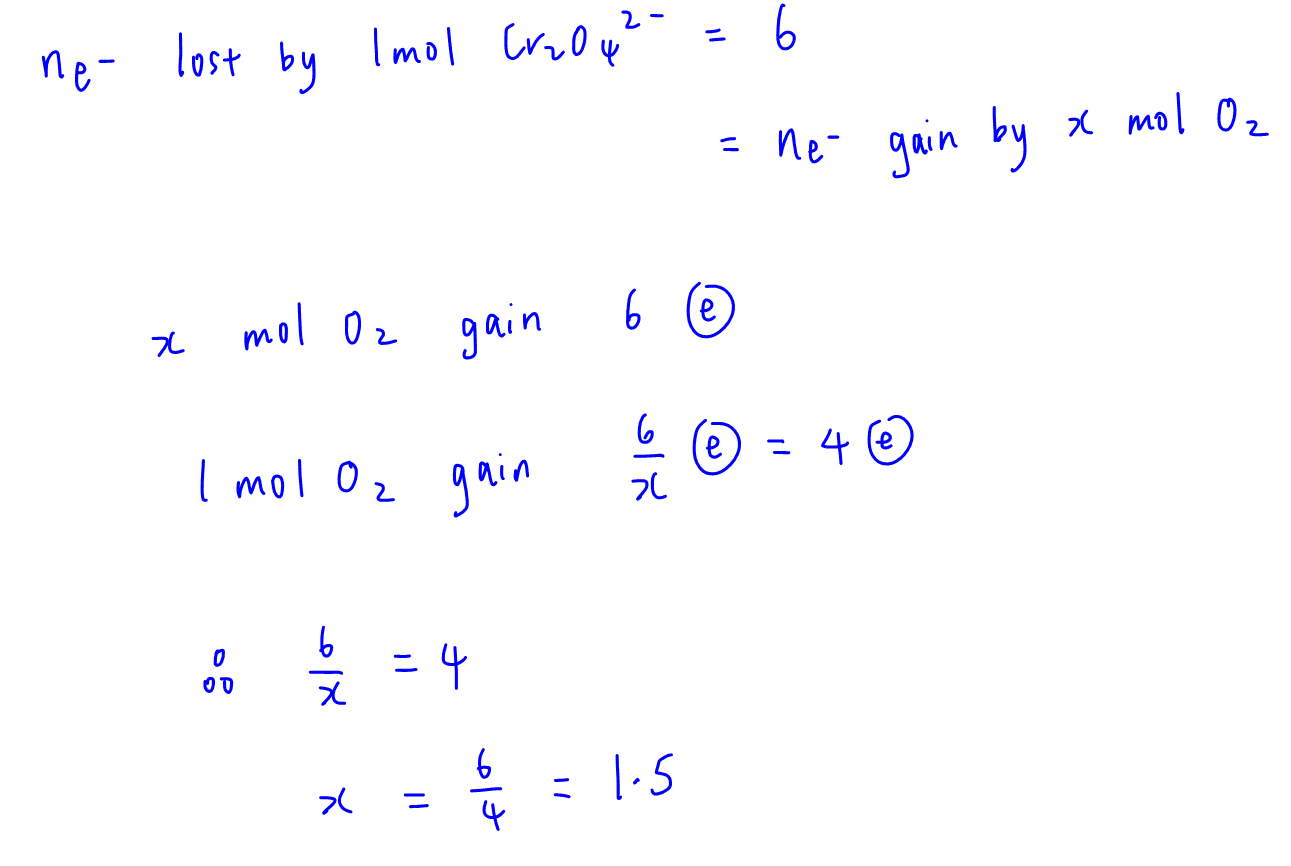
Hence we will get the same mole ratio of 3 O2 to 2 Cr2O42-.
Topic: Redox Reactions, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2021 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
