2021 P1 Q7 - Compare Boiling Point of Simple Molecules
Let Chemistry Guru, Singapore's top choice for JC Chemistry tuition, guide you through 2021 A Levels H2 Chemistry Paper 1 Question 7.
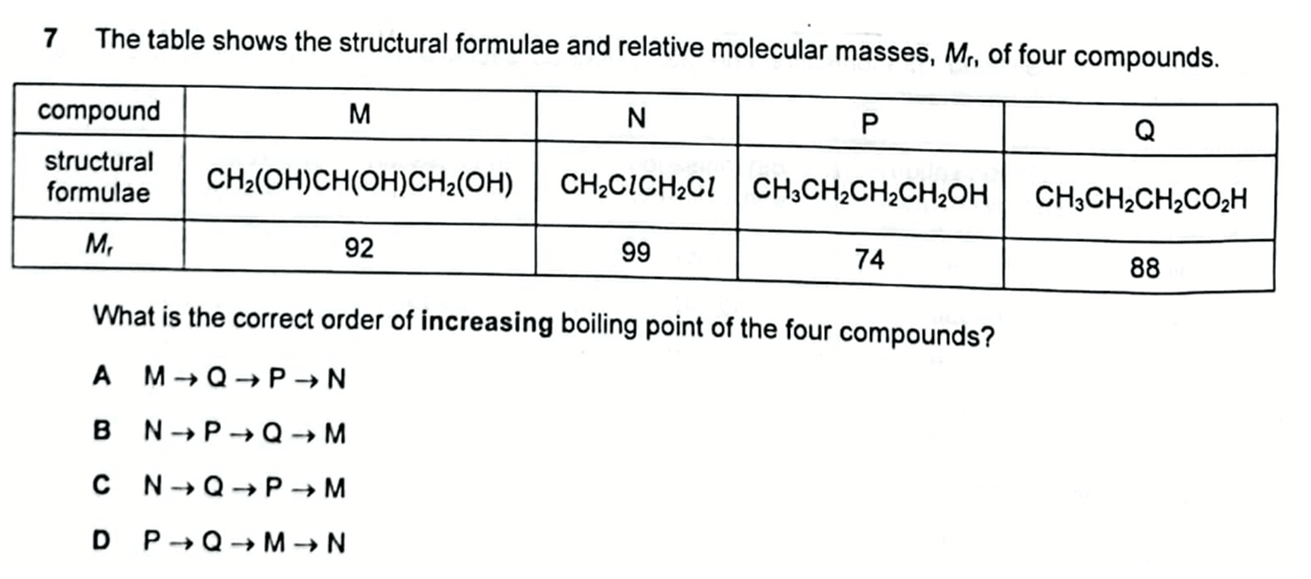
We want to arrange the above compounds in order of increasing boiling point.
Since these compounds are similar in size, we can use the following trend for comparing intermolecular forces:

Next let us deduce the dominant IMF for each compound.

M, N and Q have O-H bonds present hence they can form hydrogen bonds between molecules.
N is a chloroalkane and dominant IMF is instantaneous dipole - induced dipole attractions which is weaker than hydrogen bonds.
Hence N will have the lowest boiling point.
We can look through the options and eliminate option A and D since N has the lowest boiling point.
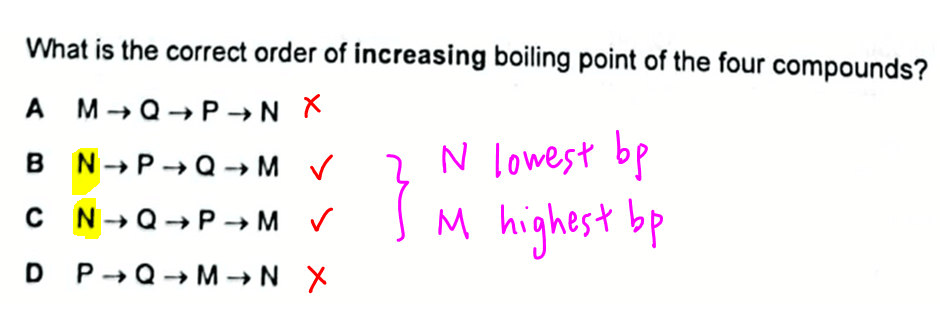
Notice based on possible answers option B and C, compound M must have the highest boiling point.
Therefore we only need to compare the extensiveness of hydrogen bonds between compounds P and Q.
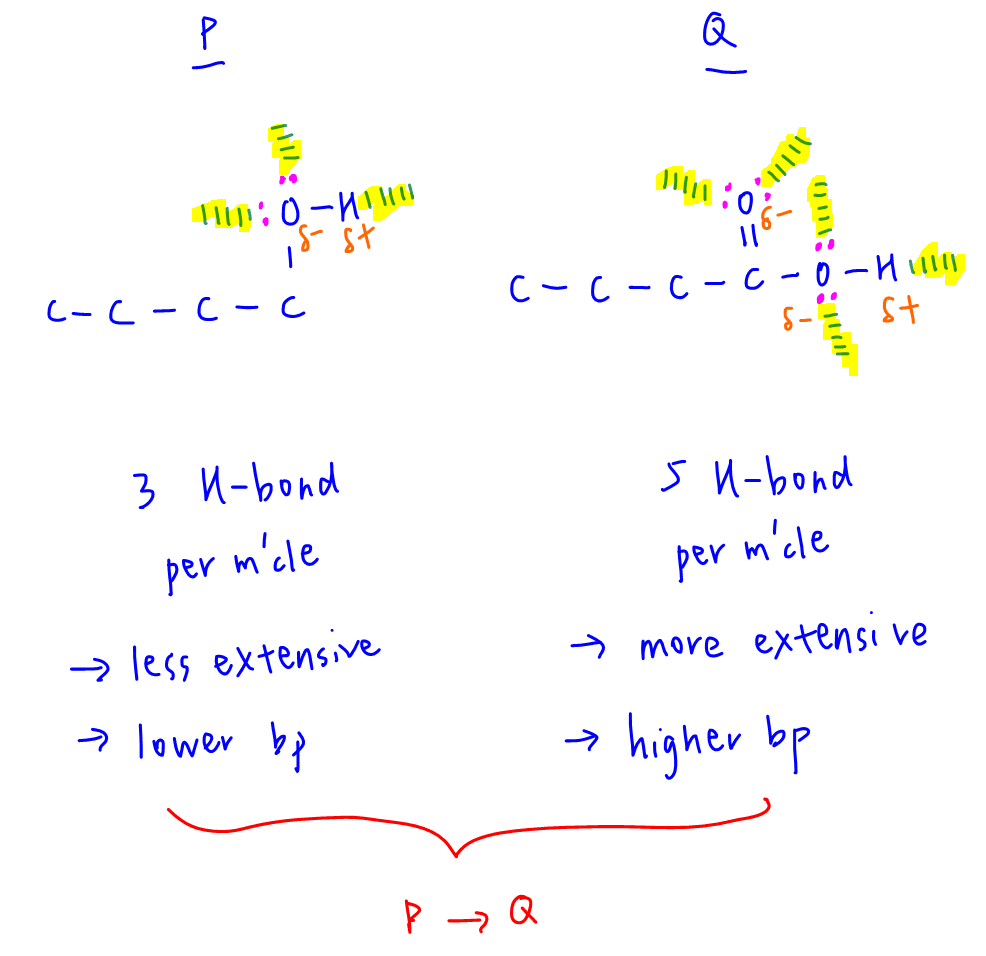
P can only form a maximum of 3 hydrogen bonds per molecule due to O-H group.
Q can form a maximum of 5 hydrogen bonds per molecule due to O-H group and additional C=O group.
Q can form more extensive hydrogen bonds, need more energy to break more H-bonds per molecule and will have higher boiling point than P.
The order of increasing boiling point will be N, P, Q and M.

Therefore the answer to this question will be option B.
We can also use extensiveness of hydrogen bond to explain why M has the highest boiling point.
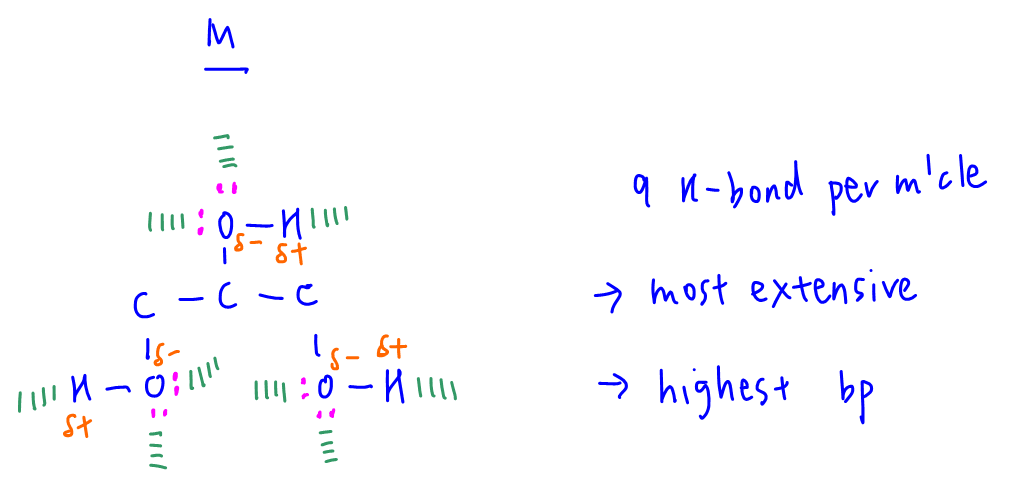
M has 3 O-H groups that can form a total of 9 hydrogen bonds per molecule.
This means M can form the most extensive hydrogen bonds amongst all compounds in this question and it will have the highest boiling point.
Topic: Intermolecular Forces, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2021 A Level H2 Chemistry Paper 1.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's esteemed H2 Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
