2022 A Level H2 Chemistry Paper 1 Solutions - Questions 21 to 30
Question 21

Answer: A
Topic: Alkene
Explanation:
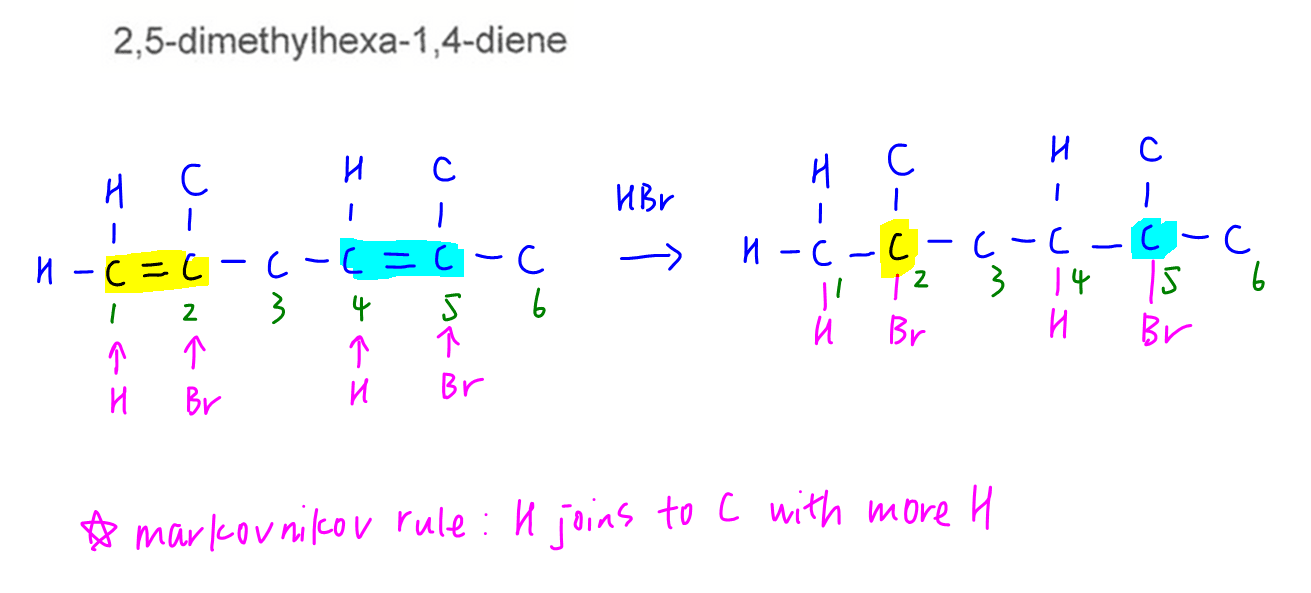
When HBr is added to both alkenes, we can use Markovnikov rule (H adds to C with more H) to deduce the major product 2,5-dibromo-2,5-dimethylhexane.
Question 22
Answer: B
Topic: Halogenoalkane
Explanation:
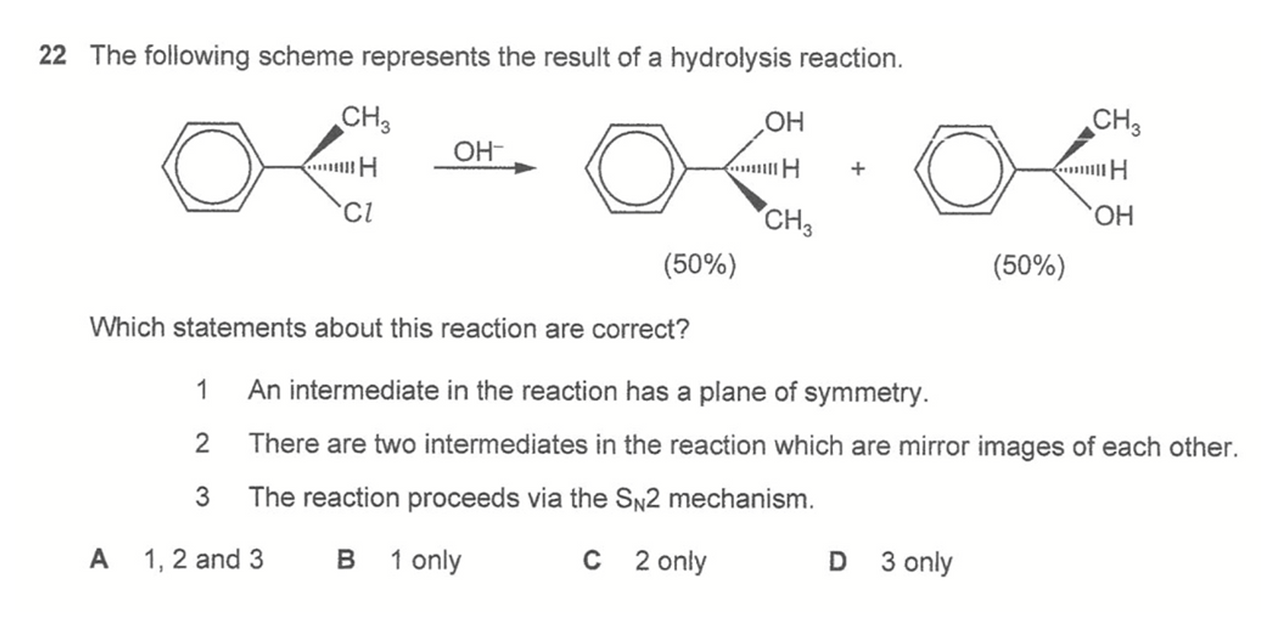
The product is a racemic mixture hence reaction is via SN1 mechanism.
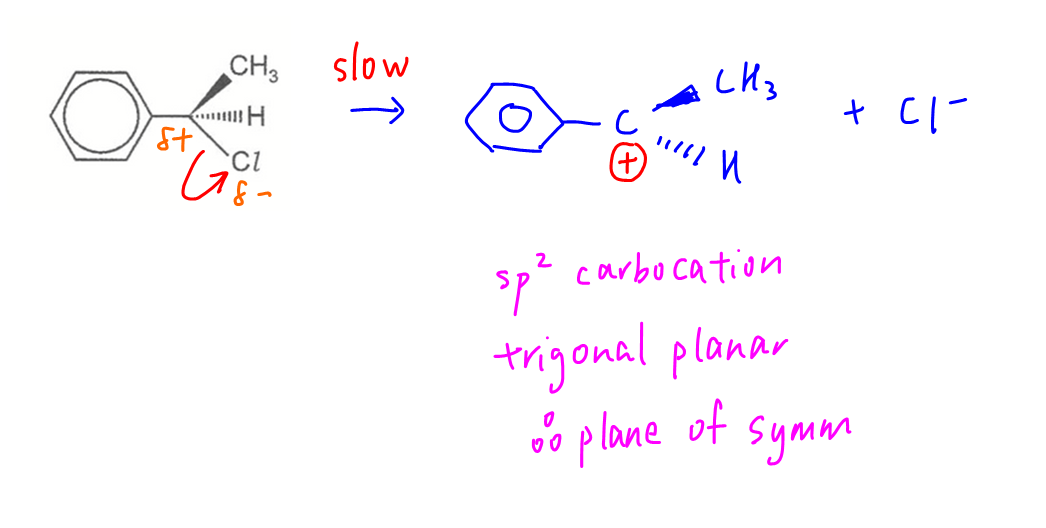
In step 1 the C-Cl bond breaks heterolytically to form a carbocation intermediate which is trigonal planar with a plane of symmetry.
Question 23

Answer: B
Topic: Halogenoalkane
Explanation:
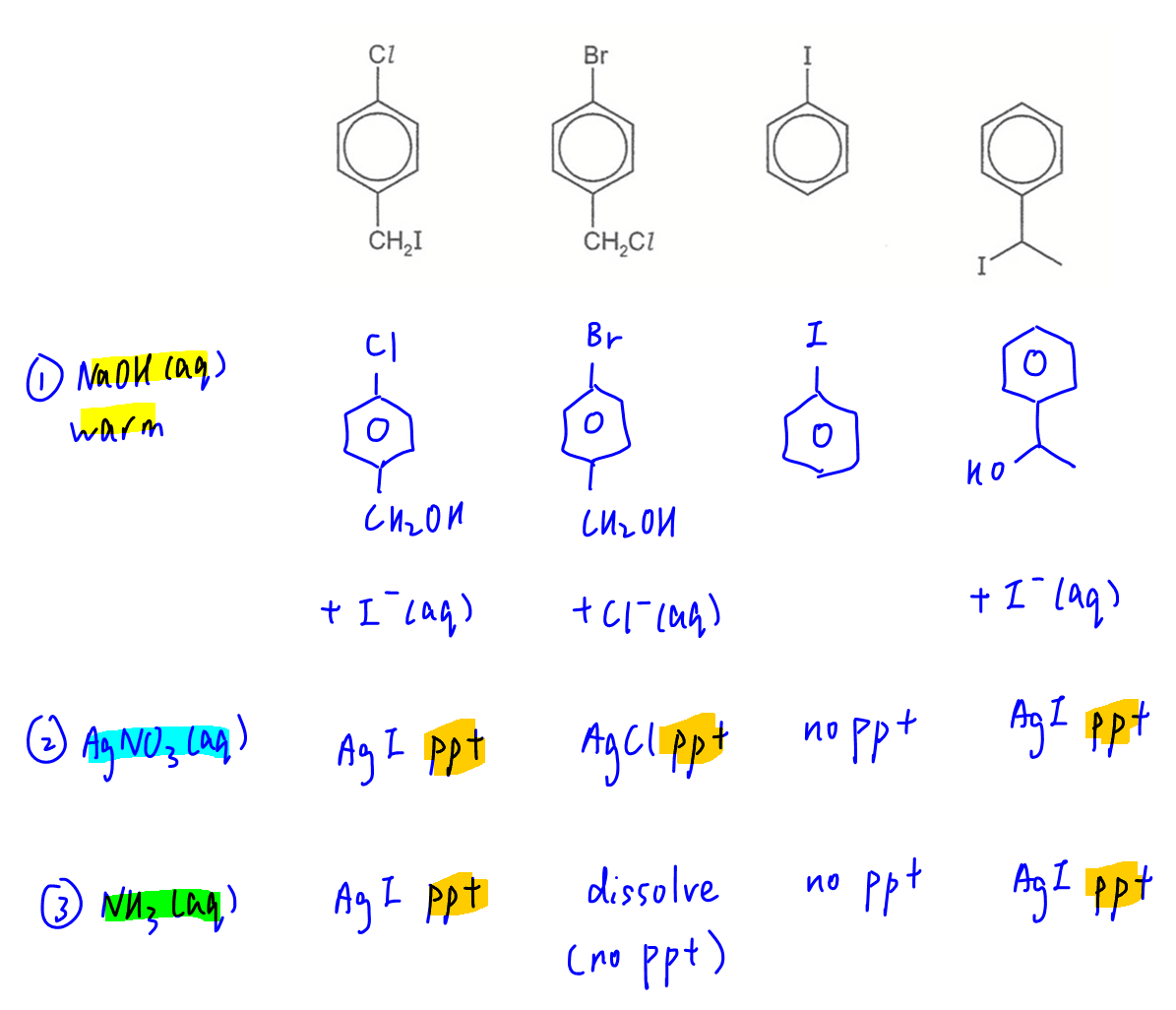
Only halogenoalkane will undergo nucleophilic substitution with NaOH.
Halogenobenzene will not react as C-X bond is stabilised by resonance.
Therefore only 3 of the compounds with halogenoalkanes will give aqueous halides that can be precipitated with AgNO3.
When shaken with ammonia solution only AgCl will dissolve.
Therefore only 2 of the compounds will have AgI ppt remaining after reaction with ammonia solution.
Question 24

Answer: B
Topic: Intro to Organic Chem
Explanation:
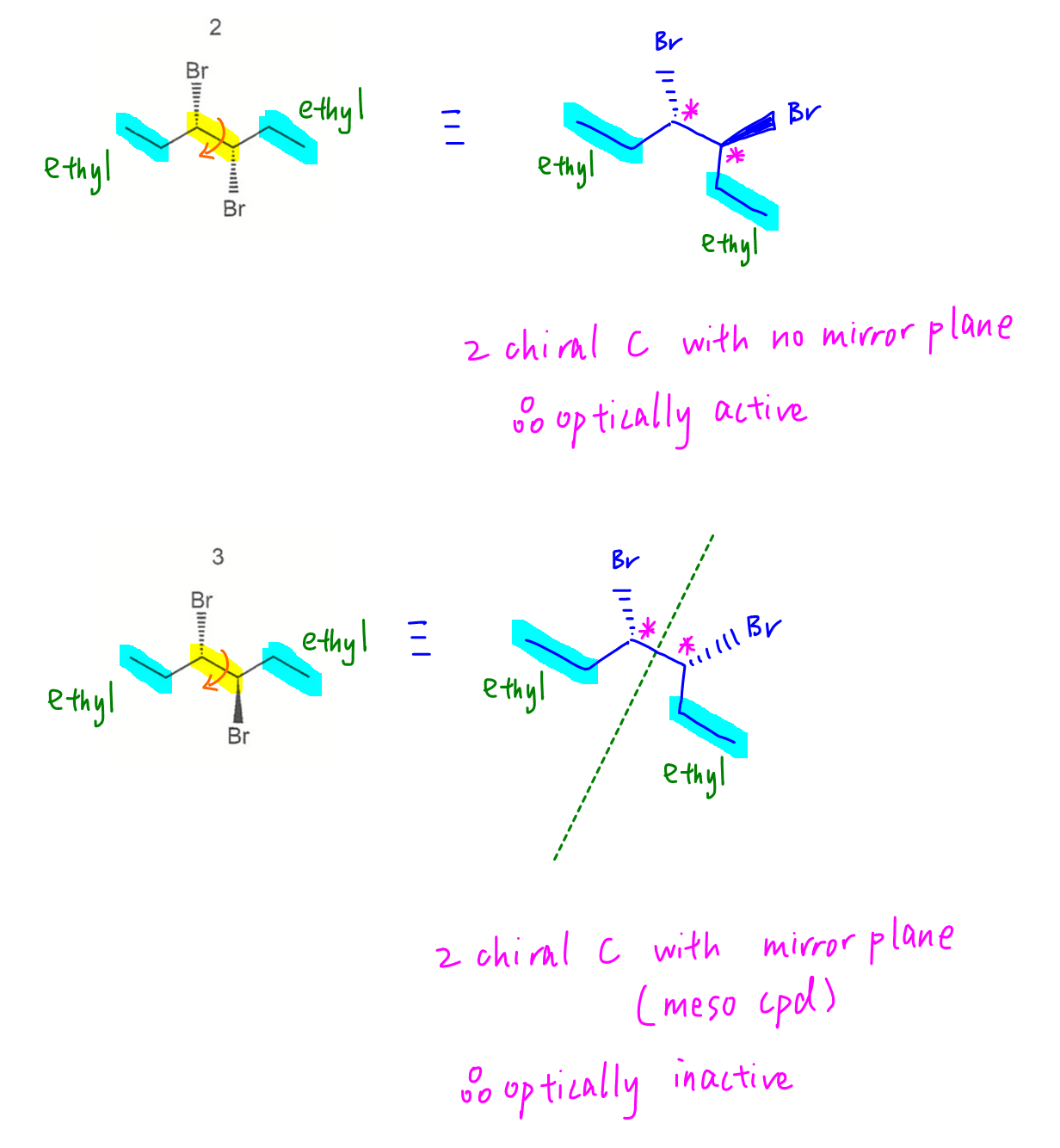
Compound 1 has chiral carbon hence optically active.
For the other compounds we can rotate C-C bond between carbons 3 and 4 so that it's easier to determine if carbons 3 and 4 are mirror images of each other.
For compound 2 there is no internal mirror plane hence it is optically active.
For compounds 3 and 4, there is an internal mirror plane hence this is considered a meso compound.
The 2 chiral carbons will cancel out each other's optical activity and hence optically inactive.
Question 25
Answer: D
Topic: Organic Synthesis
Explanation:
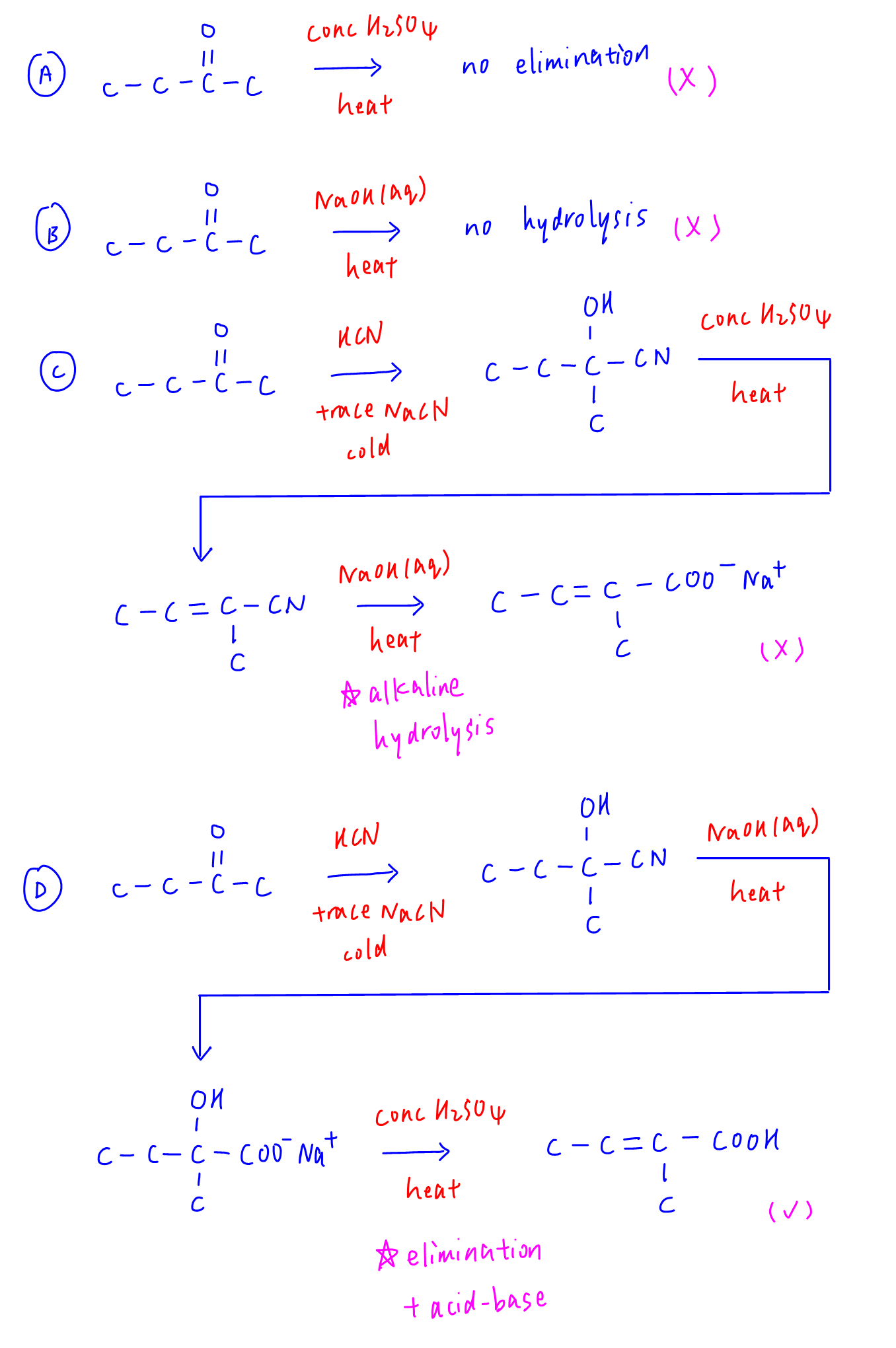
Options A and B are wrong since carbonyl compounds do not react with conc H2SO4 or NaOH(aq).
Option C will give a final product very close to the required answer, salt of conjugate base instead of acid group.
Option D is the best answer since last step with conc H2SO4 will eliminate alcohol to alkene and protonate conjugate base to carboxylic acid and give us the required final product.
Question 26

Answer: D
Topic: Carboxylic Acid
Explanation:
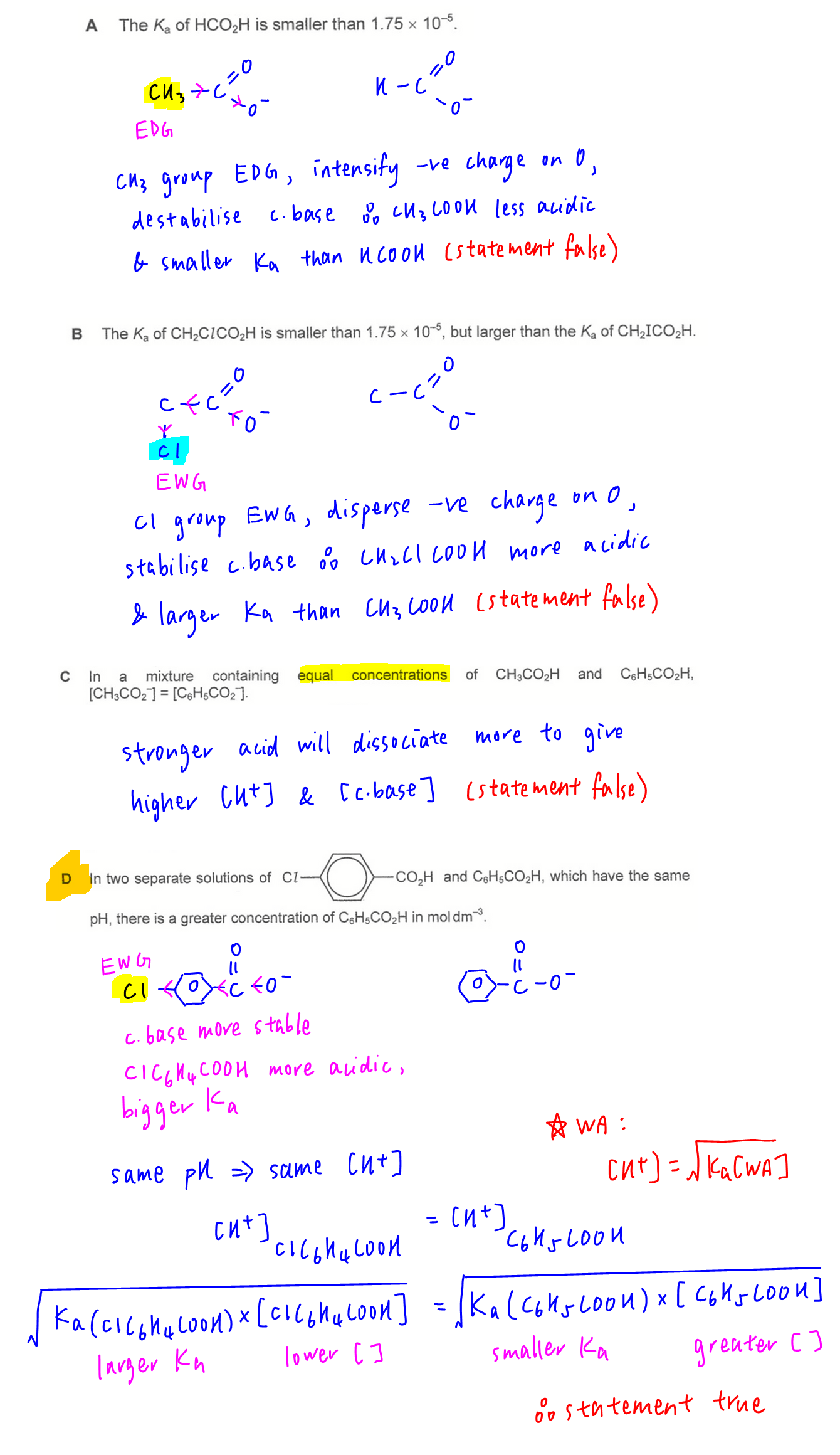
By considering stability of conjugate base, HCOOH is more acidic and has a bigger Ka value than CH3COOH, hence option A is wrong.
CH2ClCOOH is more acidic and has a bigger Ka value than CH3COOH hence option B is wrong.
Option C is wrong since acids of different strength will dissociate to different extent to form different concentrations of H+ and conjugate base.
Option D is correct since benzoic acid is weaker than 4-chlorobenzoic acid with a smaller Ka value, hence need a higher concentration of benzoic acid to dissociate same concentration of H+ to give same pH.
Question 27

Answer: A
Topic: Nitrogen Compound
Explanation:
Amine is basic and will react with acid HCl, while amide is neutral and will not react with HCl under cold conditions.

Question 28
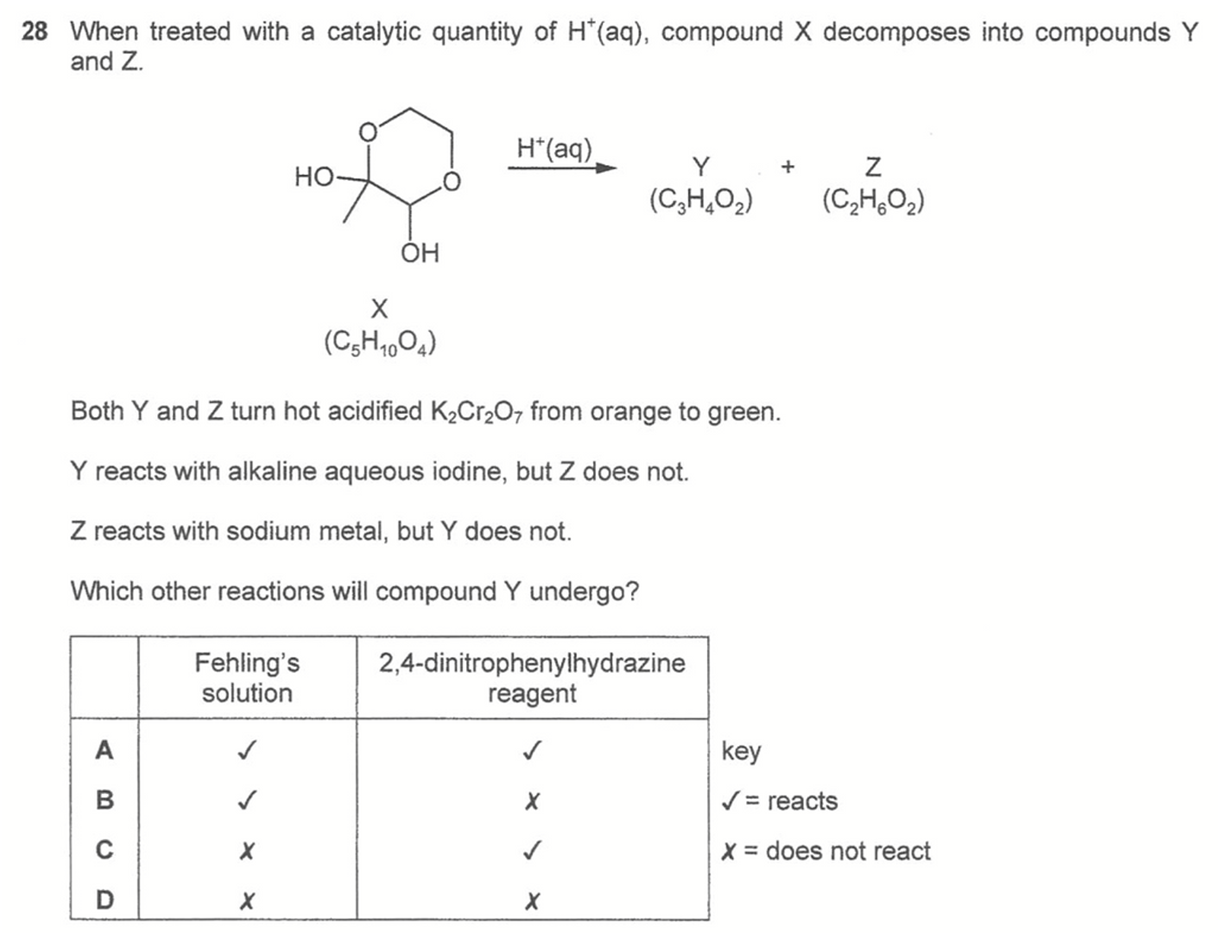
Answer: A
Topic: Carbonyl Compound
Explanation:
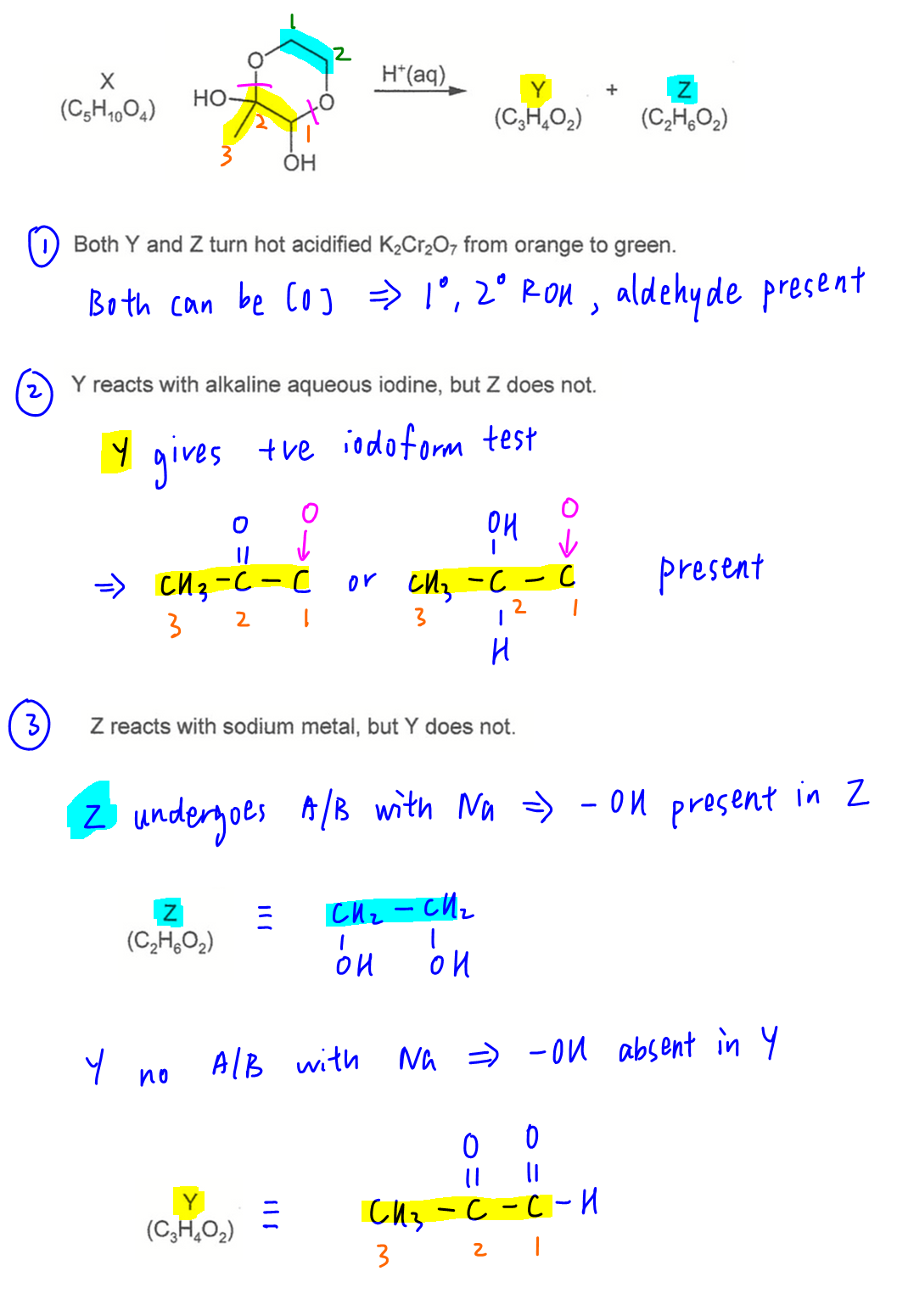
Both Y and Z can be oxidised by K2Cr2O7 hence primary alcohol, secondary alcohol or aldehyde FG present.
Y gives positive iodoform test hence will have CH3-CO-R or CH3-CHOH-R structure.
Only Z reacts with sodium hence OH group present, Z = CH2OHCH2OH
Y does not have OH group, Y = CH3COCHO
Since Y contains aldehyde, it will react with both Fehling's solution and 2,4-DNPH.
Question 29
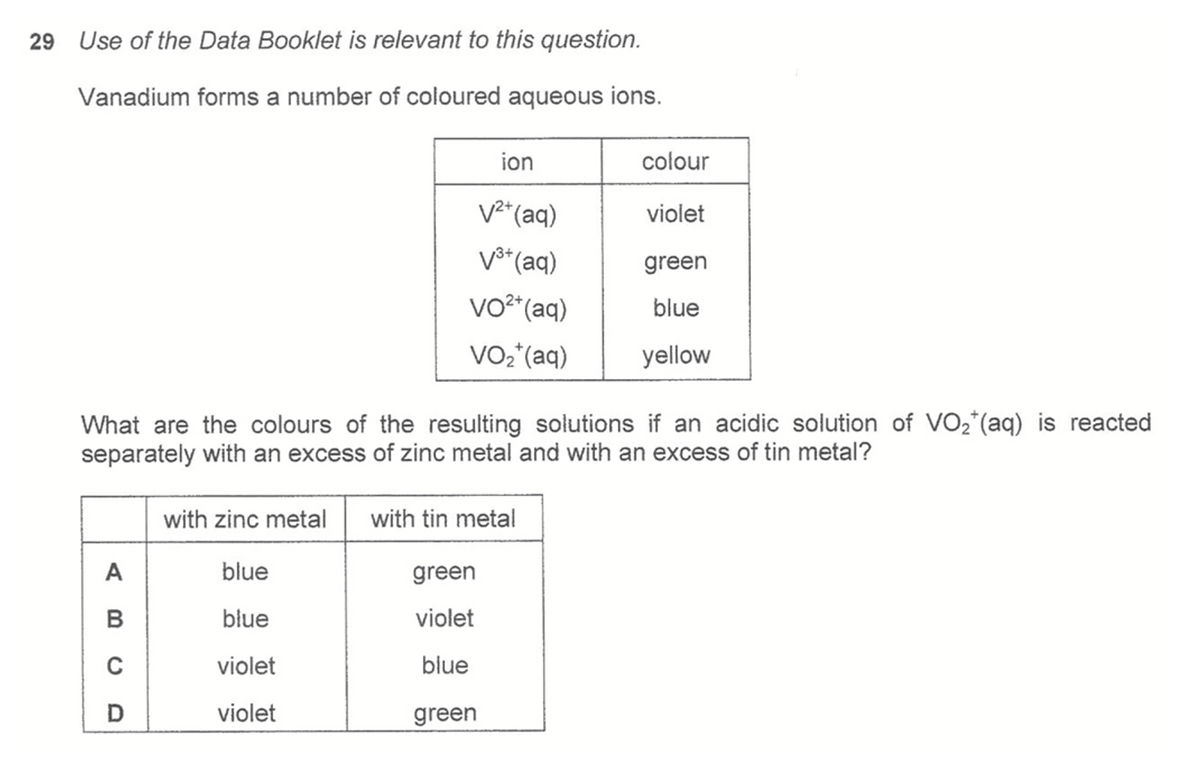
Answer: D
Topic: Electrochemistry
Explanation:
With Zn we can calculate Ecell for reaction between Zn + VO2+, Zn + VO2+ and Zn + V3+ to show that all Ecell are positive and reactions are all feasible.
Hence VO2+ will be reduced all the way to V2+, final colour is violet.
With Sn, only Ecell between Sn + V3+ is negative and reaction not feasible.
Hence VO2+ will be reduced to V3+ only, final colour is green.

Question 30

Answer: D
Topic: Transition Element
Explanation:
For transition elements the additional electrons are added to inner 3d subshell which will shield valence 4s electrons.
The increase in nuclear charge and shielding effect will cancel out and effective nuclear charge will be very similar or invariant.
This causes transition elements to have very similar physical and chemical properties such as ionisation energies, ionic radii, density, charge density and so on.
Option A and C are wrong as they are suggesting the valence 4s electrons are causing the shielding effect.
Option B is wrong since there are no electrons in 4p subshell for transition elements.

Back to other 2022 A Level Paper 1 Questions
Found this Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new Chemistry videos every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
