2022 A Level H2 Chemistry Paper 1 Solutions - Questions 11 to 20
Question 11

Answer: D
Topic: Energetics
Explanation:
Enthalpy change is negative as given in the question.
From the balanced equation, there is an increase in moles of gas, increase in degree of disorder hence entropy change is positive.
When ΔH is negative and (-TΔS) term is negative, we can deduce Gibbs free energy is always negative and reaction is always feasible or spontaneous.

Question 12
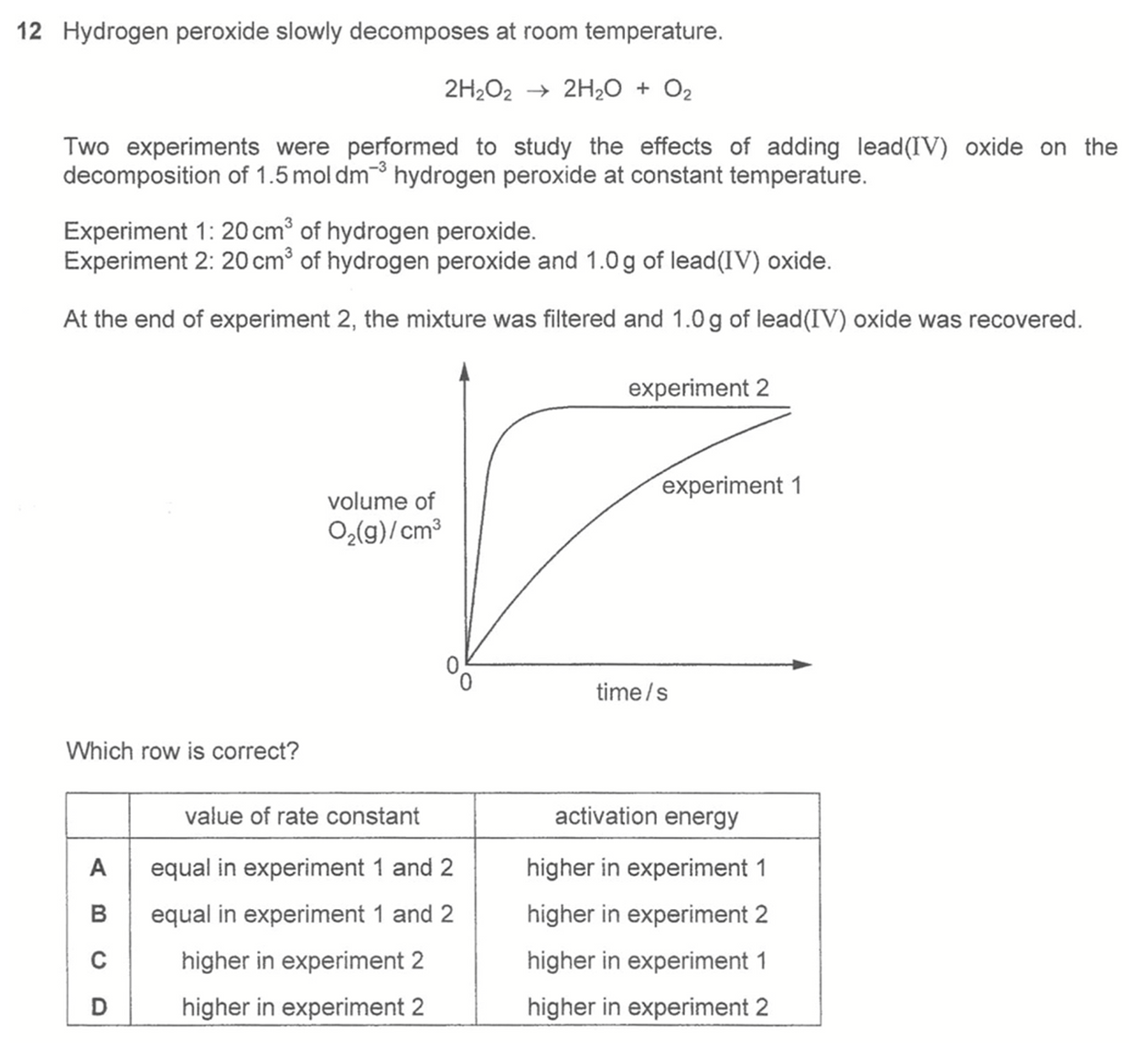
Answer: C
Topic: Kinetics
Explanation:
In experiment 2, lead(IV) oxide is added and same mass of it was recovered.
There is also an increase in rate of volume of oxygen gas collected.
This means PbO2 is a catalyst which lowers the activation energy and increase rate constant in experiment 2.

Question 13

Answer: B
Topic: Chemical Equilibria
Explanation:
We can use the ICE table to deduce equilibrium concentrations of reactants and products.
Then substitute all these values into equilibrium constant expression to solve for Kc.
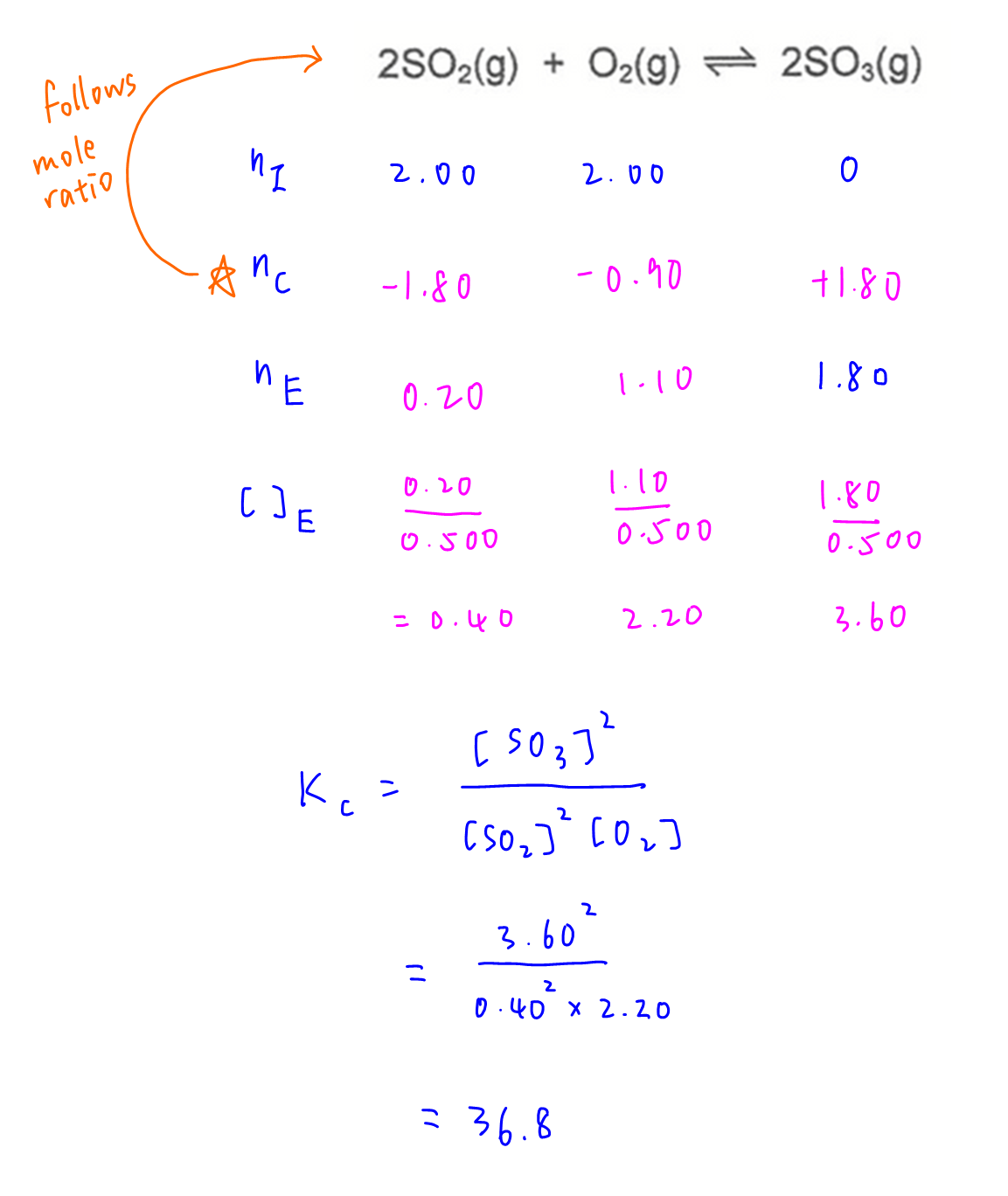
Question 14

Answer: B
Topic: Buffer Solution
Explanation:
Use buffer equation to calculate [HCO3-]/[H2CO3] = 6266
Then inverse that ratio to find [H2CO3]/[HCO3-]
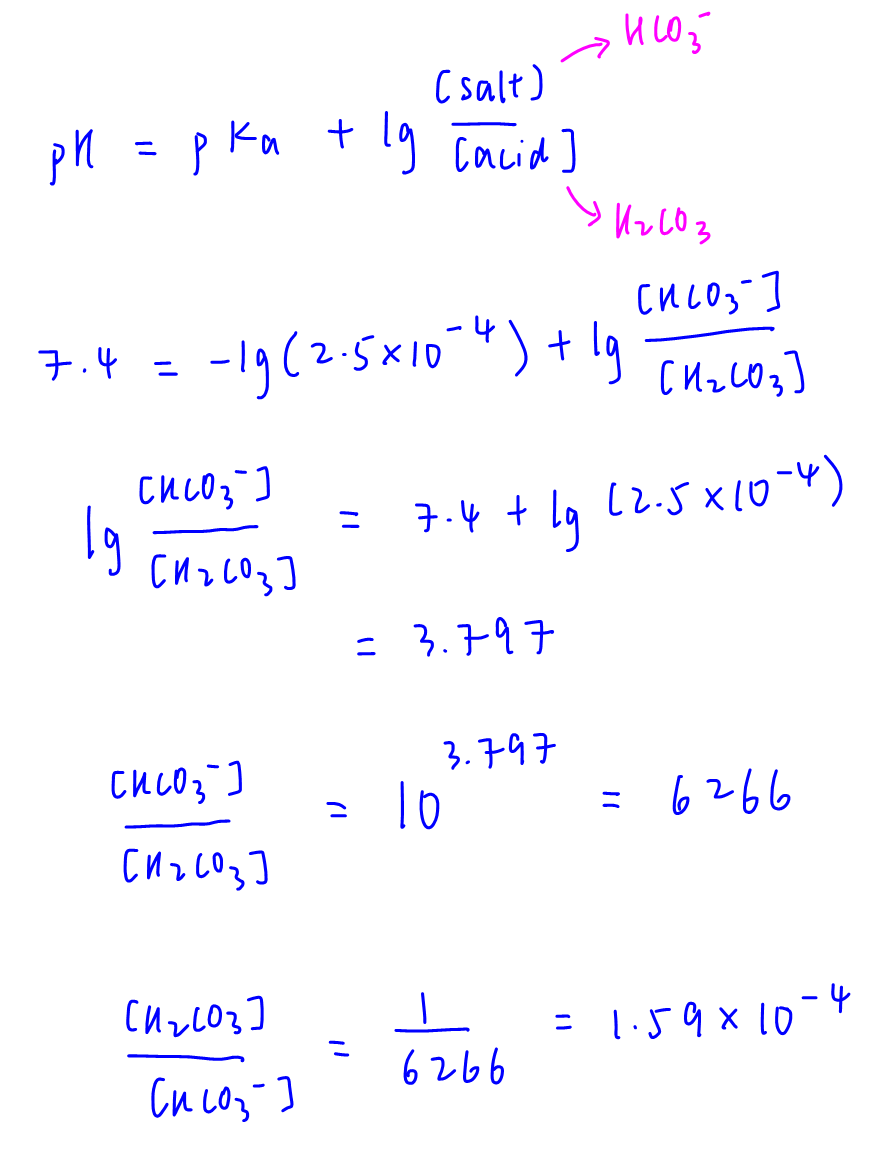
Question 15

Answer: C
Topic: Solubility Product
Explanation:
When aqueous ammonia is added, diamminesilver complex is formed which lowers concentration of Ag+(aq).
This will shift position of equilibrium to the right, cause more AgCl salt to dissolve hence solubility will increase.
When aqueous sodium chloride is added, concentration of Cl-(aq) increases.
This will shift position of equilibrium to the left, less AgCl salt will dissolve and solubility will decrease.

Question 16

Answer: C
Topic: Solubility Product
Explanation:
At equilibrium, silver ion concentration is 0.032 moldm-3 and sulfate ion concentration is 0.016 moldm-3.
We can then calculate Ksp to be 1.638 x 10-5 mol3dm-9.
In presence of 0.50 moldm-3 of Na2SO4, concentration of Ag+ is 2y, SO42- is approximately 0.50 since contribution of SO42- from sparingly soluble Ag2SO4 is negligible.
We can substitute these values into the same Ksp expression to solve for y and then silver ion concentration.

Question 17
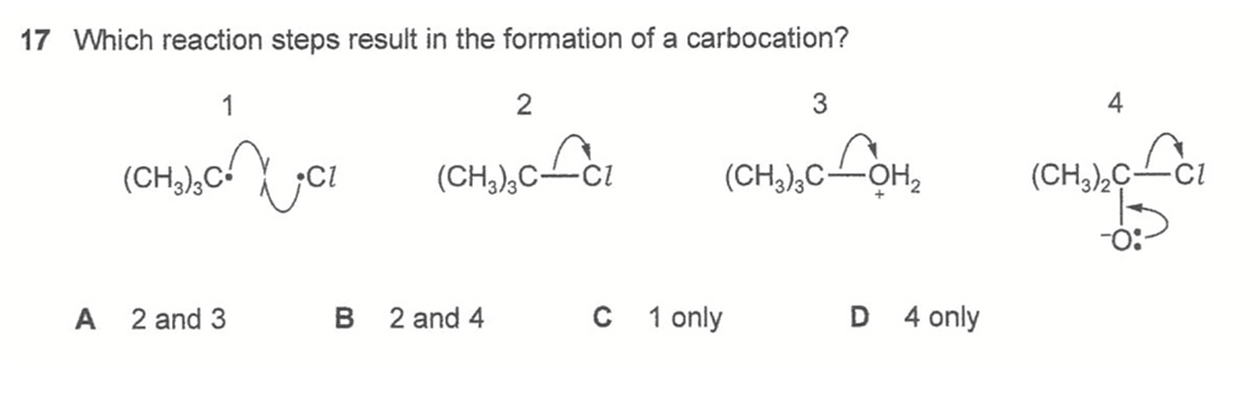
Answer: A
Topic: Organic Chemistry
Explanation:
Follow the arrow pushing to determine product and deduce which ones are carbocations.

Question 18

Answer: C
Topic: Intro to Organic Chem
Explanation:
Optical isomers will react differently with chiral compounds with optical activity.
Since only one enantiomer has the desired activity, this means the biological receptors that react with the drug must be chiral.
Enantiomers are stereoisomers with almost identical chemical and physical properties.
Only difference in physical property is the direction of rotating plane polarised light.

Question 19

Answer: B
Topic: Intro to Organic Chem
Explanation:
In the compound alkene is reduced to alkane and alcohol is oxidised to aldehyde.
The molecular formula for the reactant and product are the same, they are structural isomers of each other, hence the reaction can be considered isomerisation.

Question 20

Answer: D
Topic: Intro to Organic Chem
Explanation:
On reducing all the alkenes to alkanes, we can then find the number of chiral carbons in the product.

Back to other 2022 A Level Paper 1 Questions
Found this Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new Chemistry videos every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
