2022 A Level H2 Chemistry Paper 1 Solutions - Questions 1 to 10
Question 1

Answer: C
Topic: Chemical Bonding
Explanation:
NaF is ionic compound with strongest ionic bond between oppositely charged ions, hence highest boiling point.
CH3CH2CH3 is non-polar molecule with weak id-id attraction between molecules hence lowest boiling point.
CH3CH2NH2 and CH3CH2OH can form hydrogen bonds between molecules so boiling points are higher than CH3CH2CH3.
Comparing CH3CH2NH2 and CH3CH2OH, CH3CH2OH can form stronger H-bonds between molecules since O-H bond is more polar than N-H bond.
Therefore boiling point of CH3CH2NH2 will be lower than CH3CH2OH.

Question 2

Answer: C
Topic: Atomic Structure
Explanation:
Br has more inner shells, greater shielding effect, lower effective nuclear charge, weaker attraction between nucleus and valence electrons hence less electronegative than Cl.
Therefore in Br-Cl bond, less electronegative Br will be partial positive charge while more electronegative Cl will be partial negative.

Question 3

Answer: D
Topic: Ionic Equilibria
Explanation:
Thioacetic acid has lower pKa value hence more acidic than ethanoic acid.
Hence H+ should be more easily removed from thioacetic acid instead of ethanoic acid, Ka value for thioacetic acid is larger, and thioacetic acid should have a lower pH in both acids of same concentration.

Question 4

Answer: C
Topic: Gaseous State
Explanation:
Use Ideal Gas Equation PV = nRT to calculate moles of entonox, moles of N2O and finally mass of N2O.

Question 5

Answer: A
Topic: Periodicity
Explanation:
AlCl3 will dissolve in water to form Al3+(aq) which can hydrolyse water to release H+ due to its high polarising power.
The solution, pH = 3, is acidic enough to react with Na2CO3 to form CO2 gas.
MgCl2 will dissolve in water to form Mg2+(aq) which can also hydrolyse water, but solution is less acidic, pH = 6 and not acidic enough to react with carbonate.
NaCl only dissolves in water and does not hydrolyse water, pH = 7.

Question 6

Answer: B
Topic: Periodicity
Explanation:
Fluorine is the most electronegative element and eletronegativity decreases as we move away from F.
X is more electronegative than As and adjacent elements in same group, hence X is Phosphorus.
Y is more electronegative than X and adjacent elements in same period, hence Y is Sulfur.

Question 7

Answer: A
Topic: Mole Concept
Explanation:
Calculate mass of B2O3 in 100g, moles of B2O3 and moles of B.
Calculate mass of PbO in 100g, moles of PbO and moles of Pb.
Then compare mole ratio of Pb to B.

Question 8

Answer: B
Topic: Periodicity
Explanation:
The 4 elements mentioned in Group 15, 16 and Period 3, 4 are P, S, As and Se.
We write out the electronic configuration of M5+ for each element to deduce which subshell the 6th electron is residing.
For P, 6th electron removed from 2nd principal quantum shell, closest to nucleus, hardest to remove hence highest 6th IE.
For Se, 6th electron removed from 4th shell, furthest from the nucleus hence easiest to remove, lowest 6th IE.
Finally comparing S & As, 6th electron removed from the same 3rd shell, As will have greater ENC and 6th IE for As will be greater than S.
6th IE trend will therefore be Se < S < As < P and we can identify sulfur to be element H.

Question 9

Answer: B
Topic: Atomic Structure
Explanation:
The process is removing electron from I-, Xe and Cs+.
All species have same number of electrons hence same shielding effect with increaing nuclear charge due to increasing proton number. ENC and IE decrease in the order Cs+ > Xe > I-

Question 10

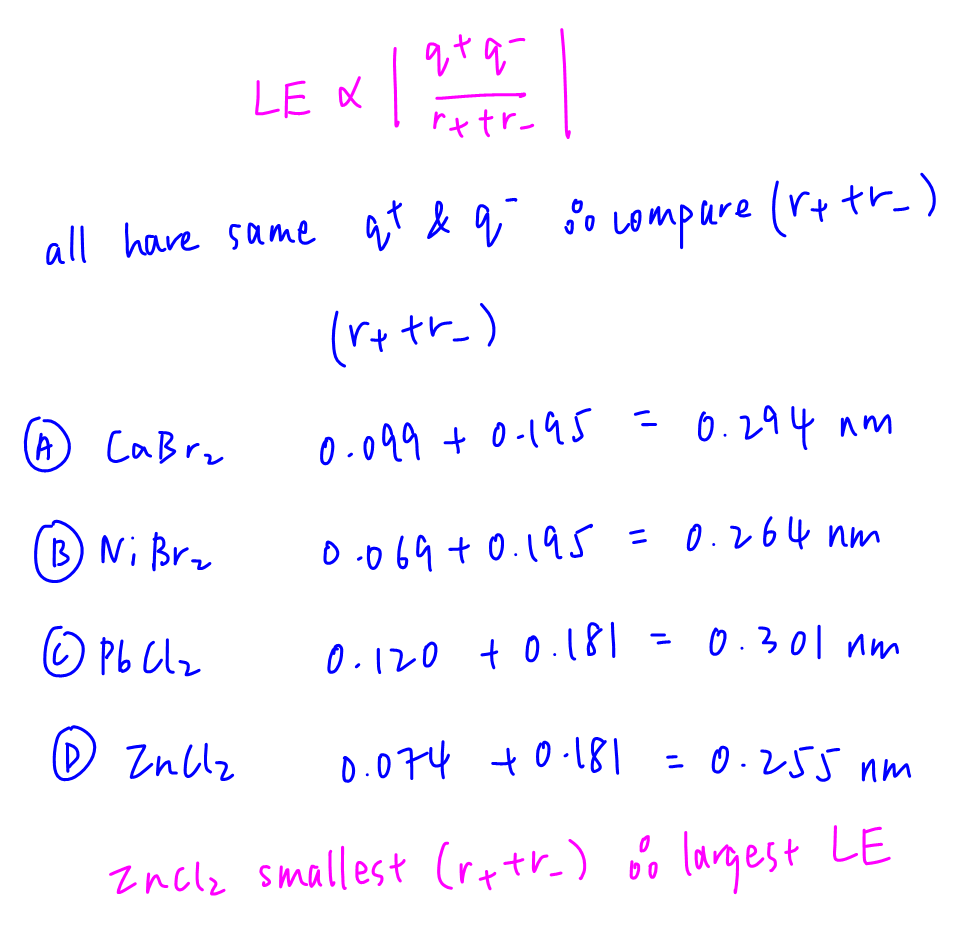
Answer: D
Topic: Chemical Bonding
Explanation:
All ionic compounds have same cation charge and anion charge, hence we just need to compare distance between the cation and anion (r+ + r-)
ZnCl2 has the smallest (r+ + r-) term hence largest lattice energy.
Back to other 2022 A Level Paper 1 Questions
Found this Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new Chemistry videos every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
