2023 A Level H2 Chemistry Paper 1 Solutions - Questions 11 to 20
Question 11
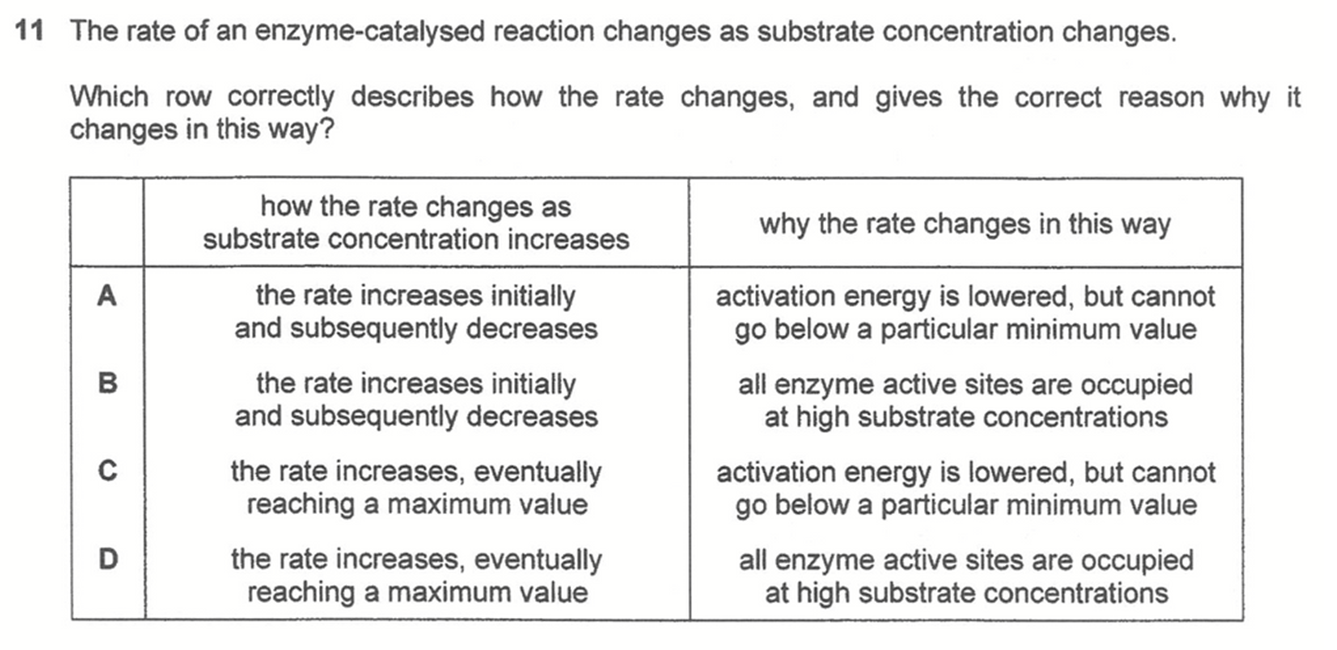
Answer: D
Topic: Kinetics
Explanation:
When substrate concentration is lower than enzyme concentration, there is enough enzyme to handle the increase in concentration of substrate. Hence rate will be first order with respect to substrate and rate increases as substrate concentration increases.
When substrate concentration is higher than enzyme concentration, saturation occurs where all enzymes are fully utilised so rate of reaction will reach a maximum and stay constant, ie zero order with respect to substrate.
Question 12

Answer: D
Topic: Chemical Equilibria
Explanation:
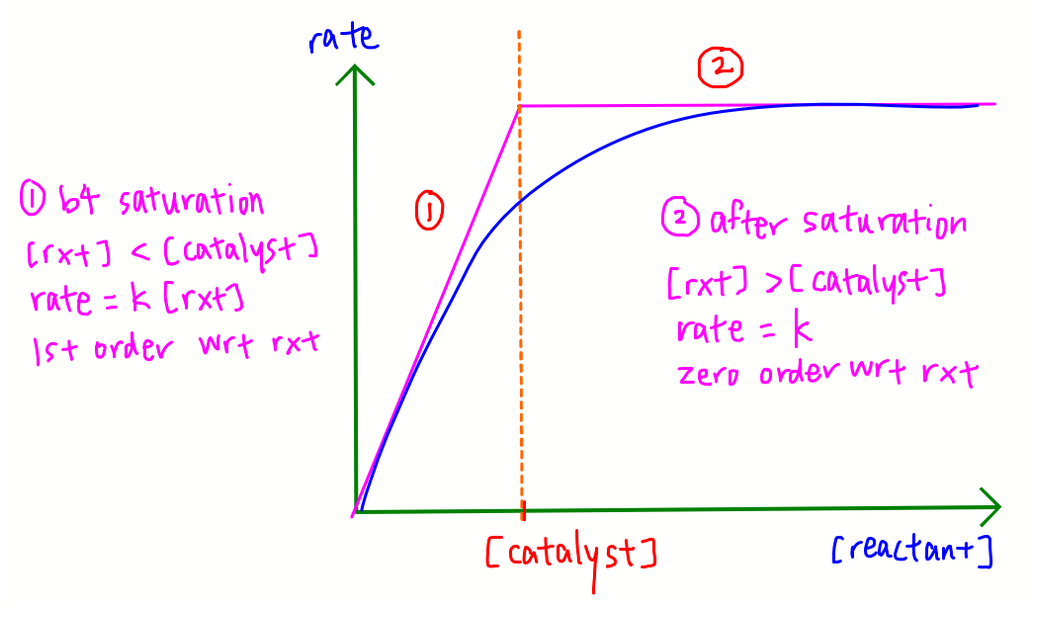
Equilibrium constant K is only affected by changes in temperature hence adding catalyst or changing pressure will not change K.
For exothermic reaction, when there is increase in temperature, position of equilibrium will shift left to favour endothermic reaction and absorb excess heat. Partial pressure of reactant will increase while partial pressure of product will decrease, hence Kp will decrease.
Question 13

Answer: C
Topic: Chemical Equilibria
Explanation:
Let initial partial pressures of N2 and H2 be x and 3x. We can then fill up the ICE table to determine equilibrium partial pressures in terms of x.
P(N2) = (x - 46); P(H2) = (3x - 138); P(NH3) = 92
Total pressure is 100 atm hence we can solve for x = 48 and determine equilibrium partial pressures to be
P(N2) = 2; P(H2) = 6; P(NH3) = 92
Finally we can substitute these terms into Kp expression and solve for Kp = 19.6
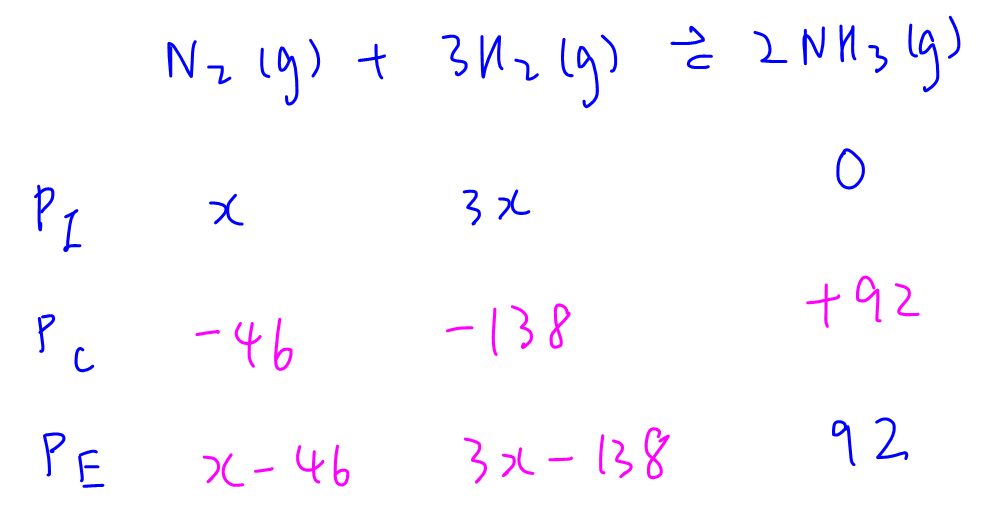
Question 14

Answer: C
Topic: Redox Titration
Explanation:
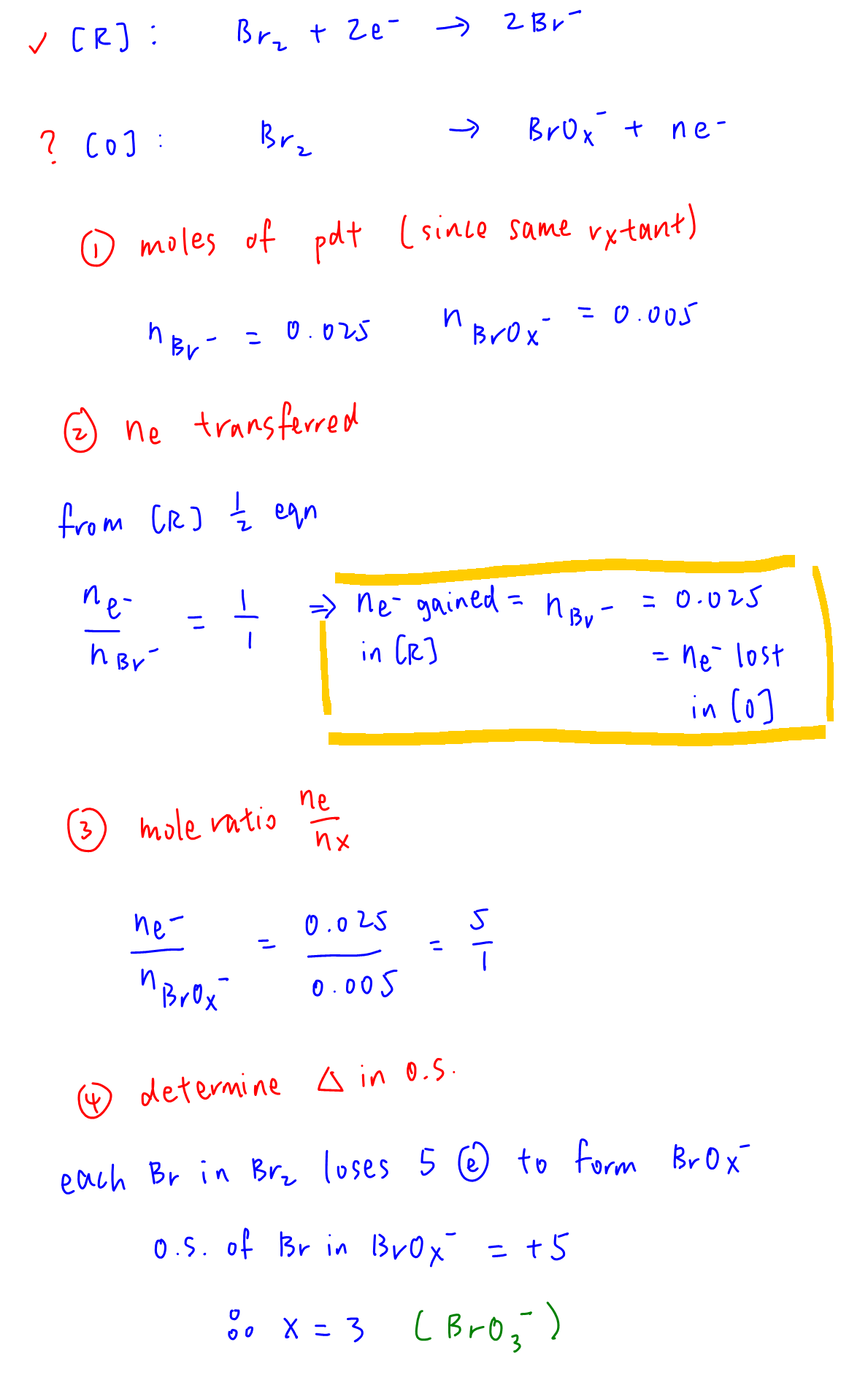
Moles of Br in reactant Br2 = 0.03; moles of Br in product KBr = 0.025
Hence moles of Br in product BrOx- = 0.03 - 0.025 = 0.005
This redox reaction is involving reduction of Br2 to Br- (known half equation) and oxidation of Br2 to BrOx- (unknown half equation).
From known reduction half equation, moles of electron gained in reduction = moles of Br- = 0.025 = moles of electron lost in oxidation.
Mole ratio of electron lost to BrOx- = 0.025 / 0.005 = 5 / 1
This means each Br in Br2 loses 5 electrons to form BrOx-, oxidation state of Br is in BrOx- is +5, hence x = 3
Question 15

Answer: B
Topic: Solubility Product
Explanation:
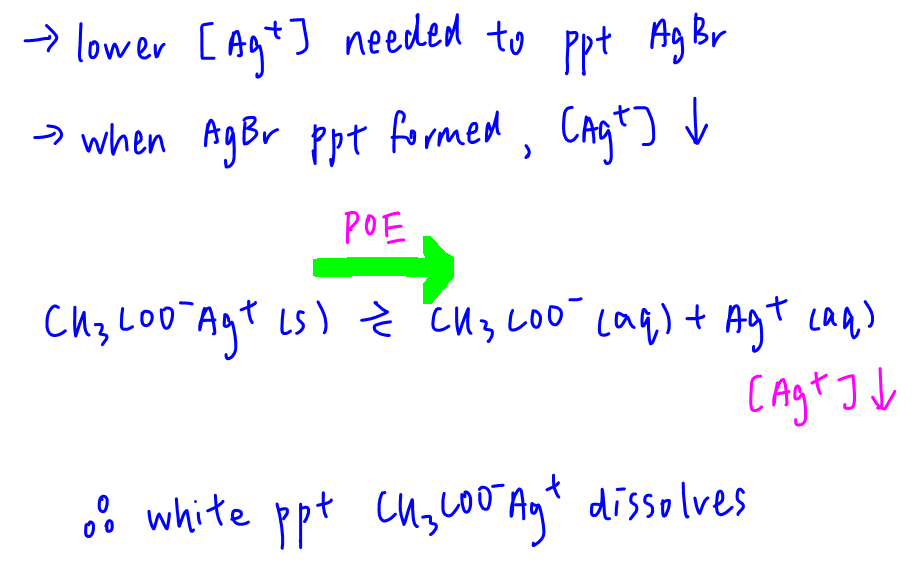
Statement 1 is correct since white ppt silver ethanoate is formed.
Statement 2 is correct since formation of cream ppt silver bromide is favoured. As AgBr is less soluble, a lower concentration of Ag+ is needed to precipitate AgBr. As AgBr ppt is formed, Ag+ concentration decreases. This will shift the position of equilibrium of dissociation of silver ethanoate to the right hence more silver ethanoate will dissolve.
Question 16

Answer: C
Topic: Intro to Organic Chem
Explanation:
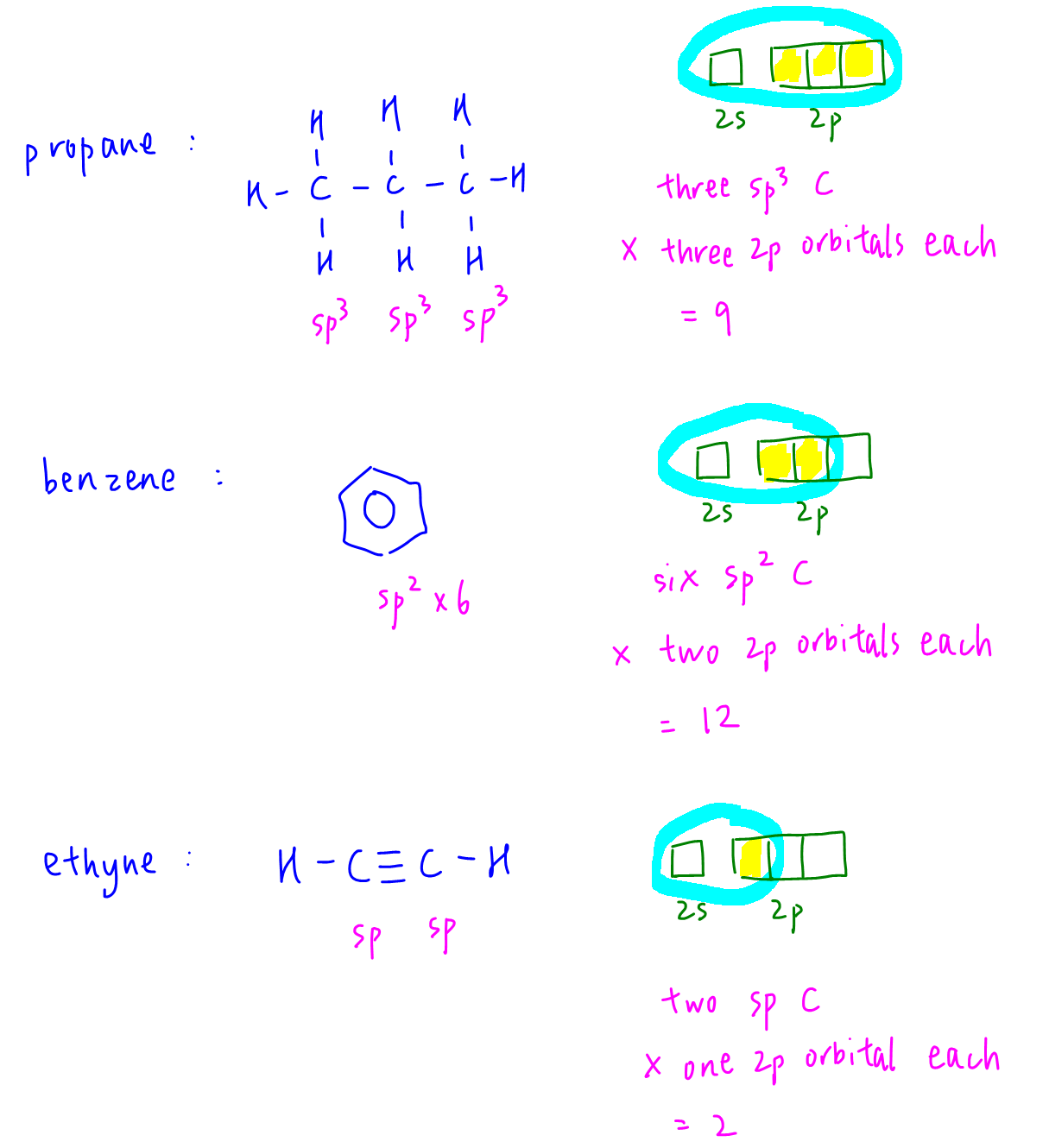
Propane has 3 sp3 hybridised carbons. Each sp3 carbon has three 2p orbitals hybridised hence total 9 2p orbitals.
Benzene has 6 sp2 hybridised carbons. Each sp2 carbon has two 2p orbitals hybridised hence total 12 2p orbitals.
Ethyne has 2 sp hybridised carbons. Each sp carbon has one 2p orbitals hybridised hence total 2 2p orbitals.
Therefore total 2p orbitals hybridised = 9 + 12 + 2 = 23.
Question 17

Answer: C
Topic: Halogenoalkane
Explanation:
For chloroethene, lone pair on Cl can interact with pi bond in ethene hence there is delocalised pi system between carbons and chlorine. There is resonance stability for C-Cl bond therefore chloroethene is unreactive to nucleophiles.
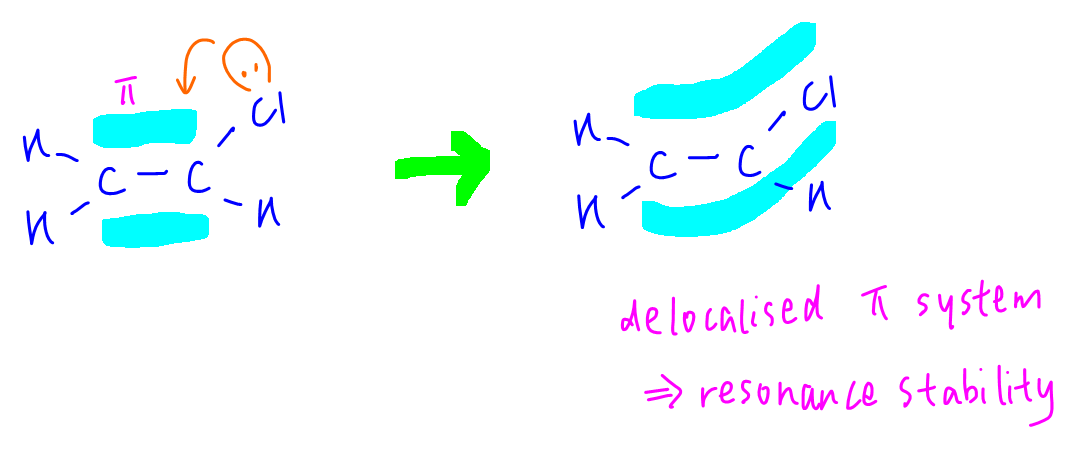
Question 18

Answer: C
Topic: Intro to Organic Chem
Explanation:
We only need to consider alkene functional groups and ignore cycloalkane since there is no ring structure. Constitutional isomers will also exclude cis-trans isomers.
A systematic permutation is required to figure out the total number of isomers = 5.
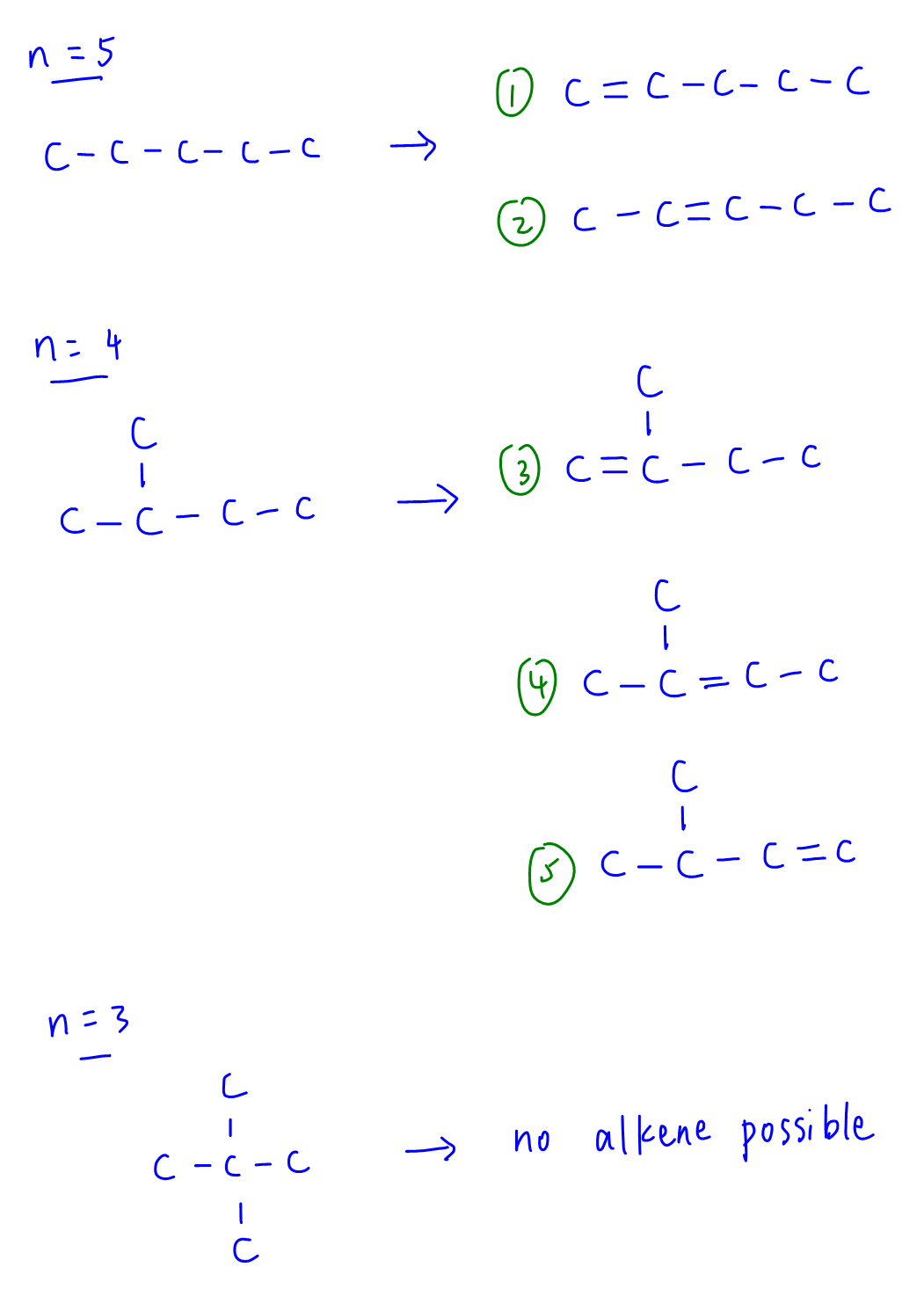
Question 19
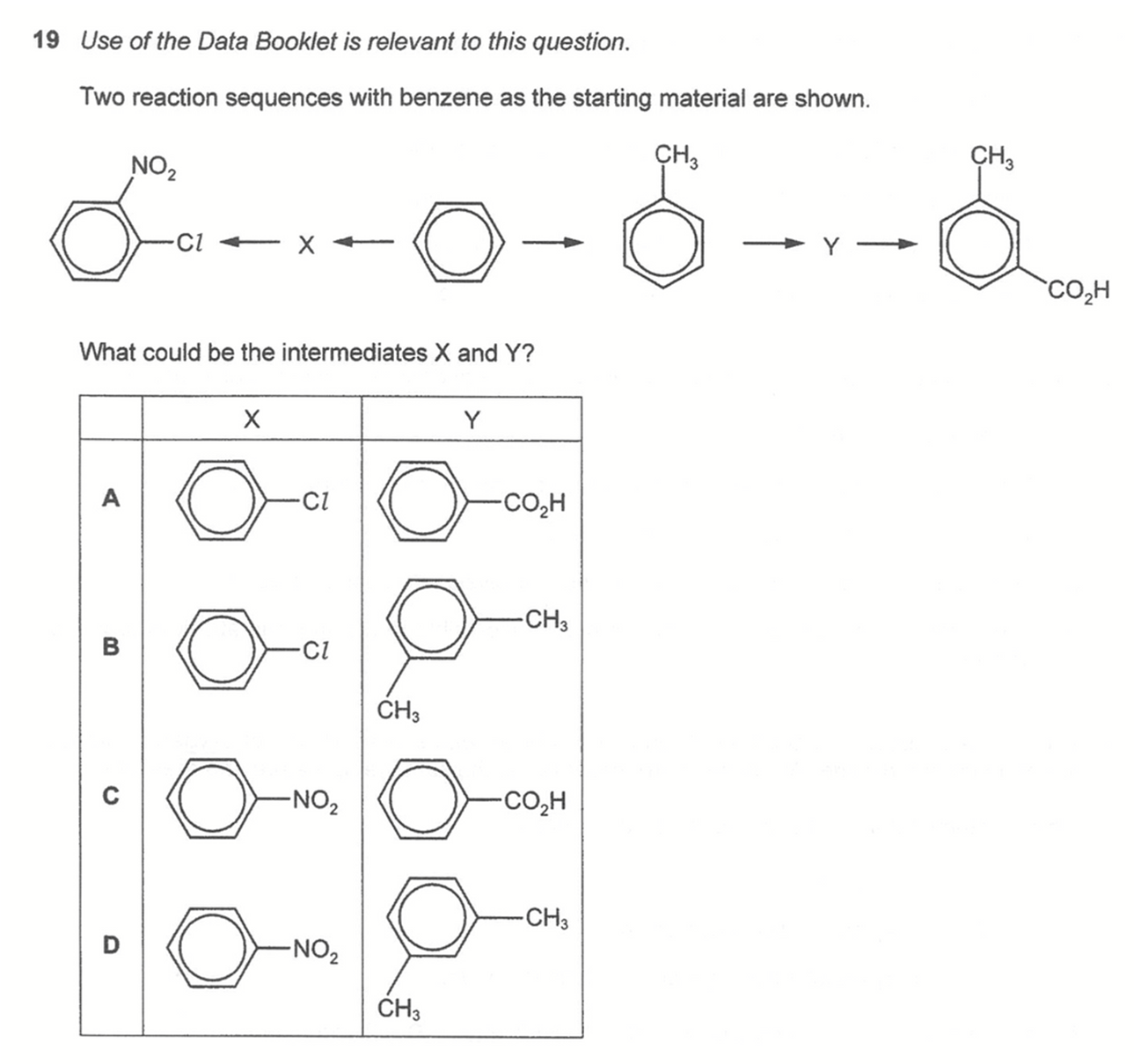
Answer: A
Topic: Benzene
Explanation:
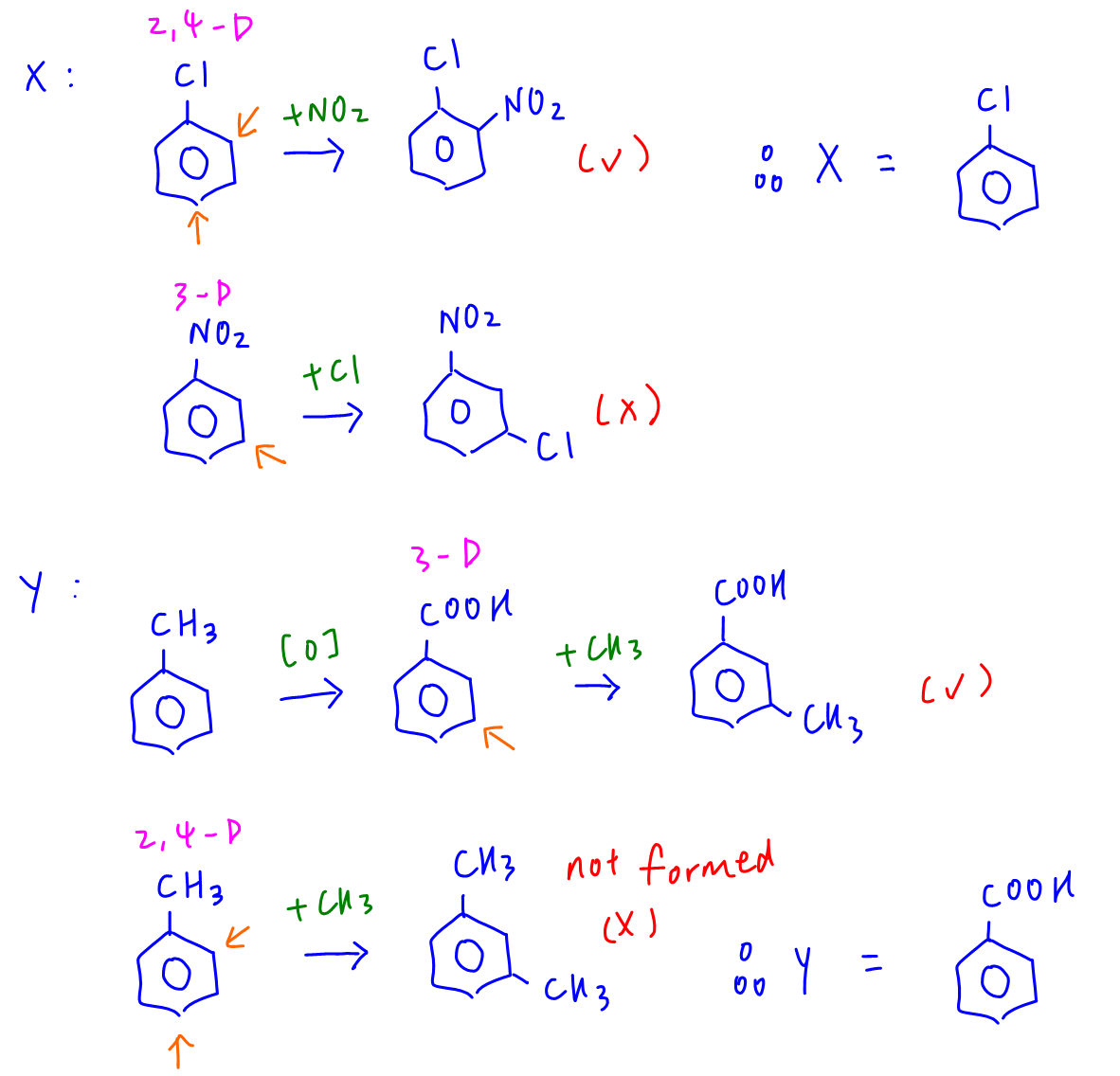
X is chlorobenzene since Cl group is 2,4 - directing and can direct nitro group to position 2 wrt Cl to form required product.
Y is benzoic acid since acid group is 3 - directing and can direct methyl group to position 3 wrt acid group to form required product.
Question 20

Answer: C
Topic: Benzene
Explanation:
Statement A is wrong as benzene undergoes electrophilic substitution.
Statement B is wrong as benzene is stable and does not take part in addition reactions without catalysts.
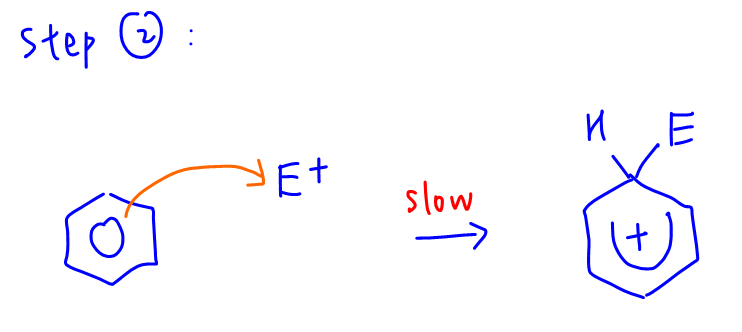
Statement C is the answer where second step for electrophilic substitution of benzene involves benzene reacting with electrophile to form intermediate.
Statement D is wrong as all the C-C bonds in benzene are equal in length and energy.
Back to other 2023 A Level Paper 1 Questions
Found this Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new Chemistry videos every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
