2023 A Level H2 Chemistry Paper 1 Solutions - Questions 1 to 10
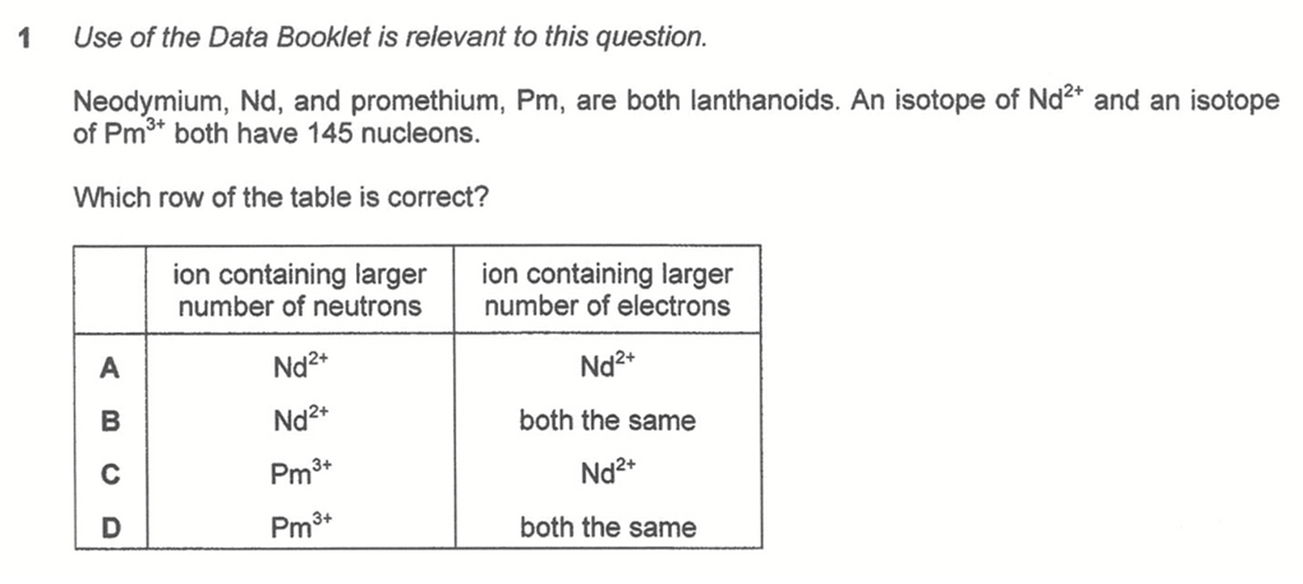
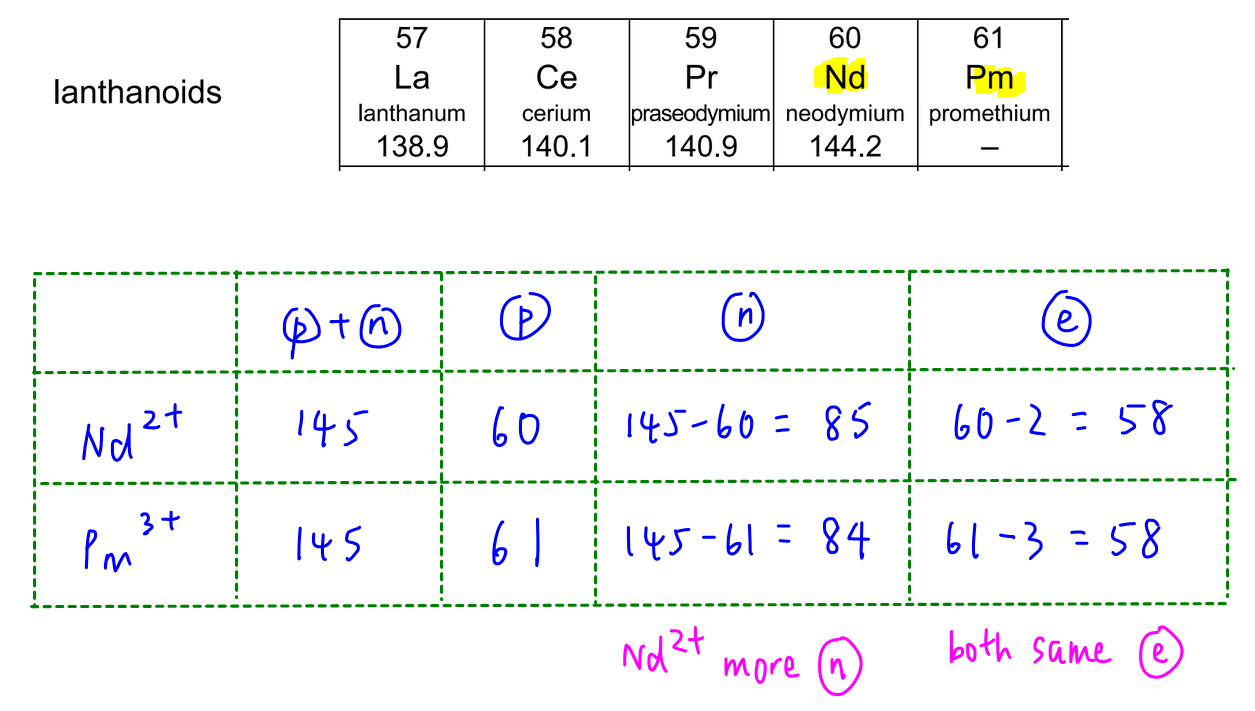
Question 1
Answer: B
Topic: Atomic Structure
Explanation:
Lanthanoids neodymium, Nd, has 60 protons while promethium, Pm, has 61 protons.
We can determine Nd2+ has 85 neutrons and 58 electrons while Pm3+ has 84 neutrons and 58 electrons.
Hence Nd2+ has more neutrons and both ions have the same number of electrons.
Question 2

Answer: C
Topic: Chemical Bonding
Explanation:
The bigger the difference in electronegativity between 2 atoms in a bond, the stronger the ionic character.
Hence YCl has greater ionic character than ZO2 since Y-Cl bond has a greater difference in electronegativity than Z-O bond.

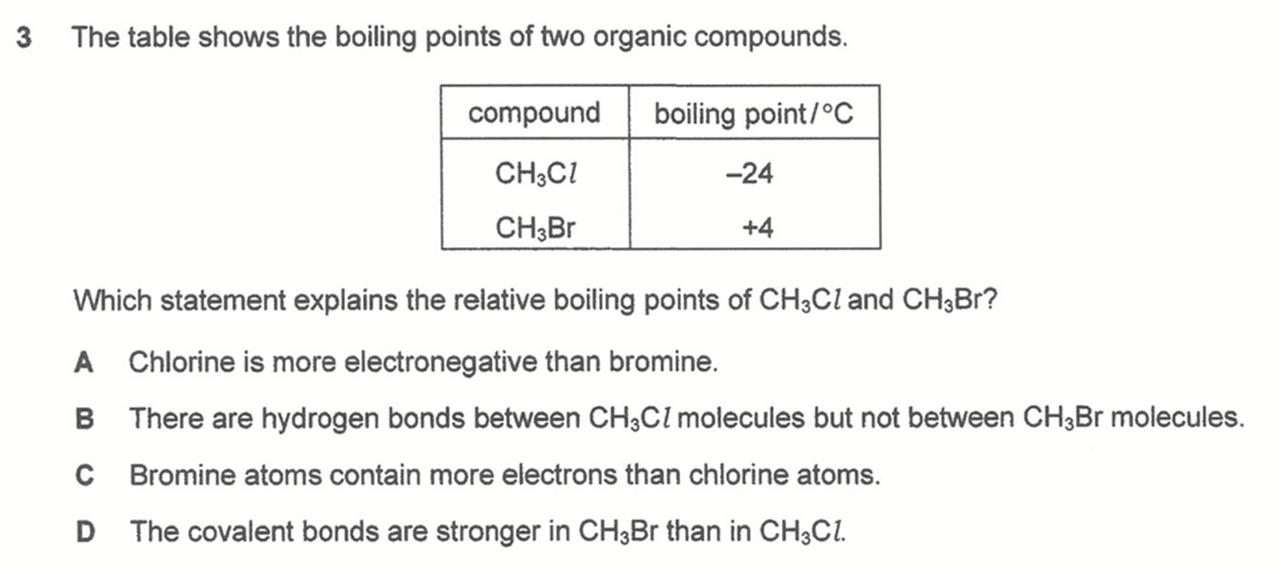
Question 3
Answer: C
Topic: Chemical Bonding
Explanation:
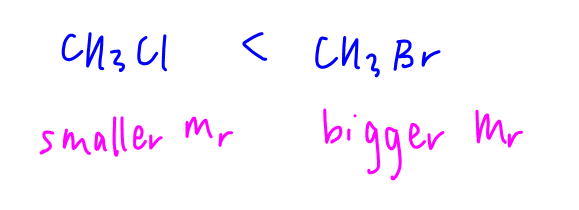
Bromine atom has more electrons than chlorine atom, CH3Br has a bigger and more polarisable electron cloud, stronger instantaneous dipole - induced dipole attraction between molecules, need more energy to overcome hence higher boiling point than CH3Cl.
Question 4
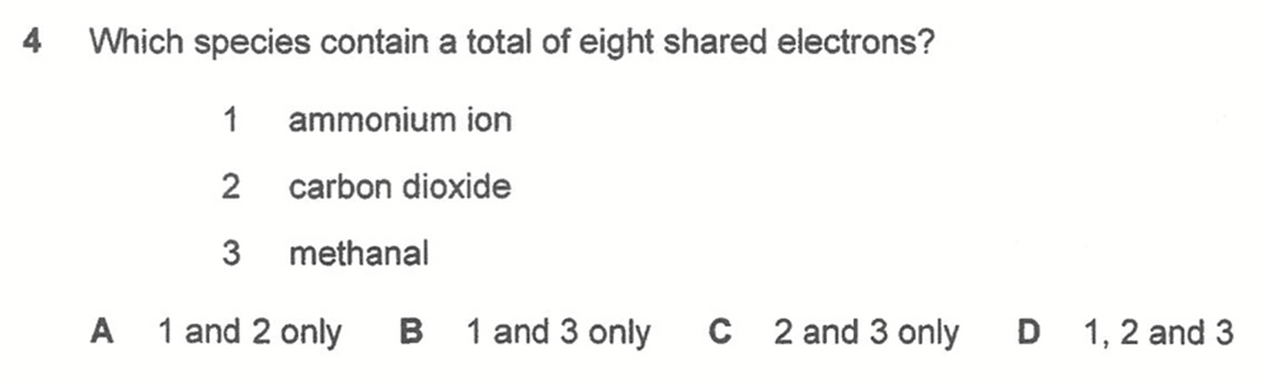
Answer: D
Topic: Chemical Bonding
Explanation:
By considering the number of electrons around central atom via their dot-cross diagrams, we can determine nitrogen in NH4+, carbon in CO2 and carbon in HCHO all have 8 shared electrons.
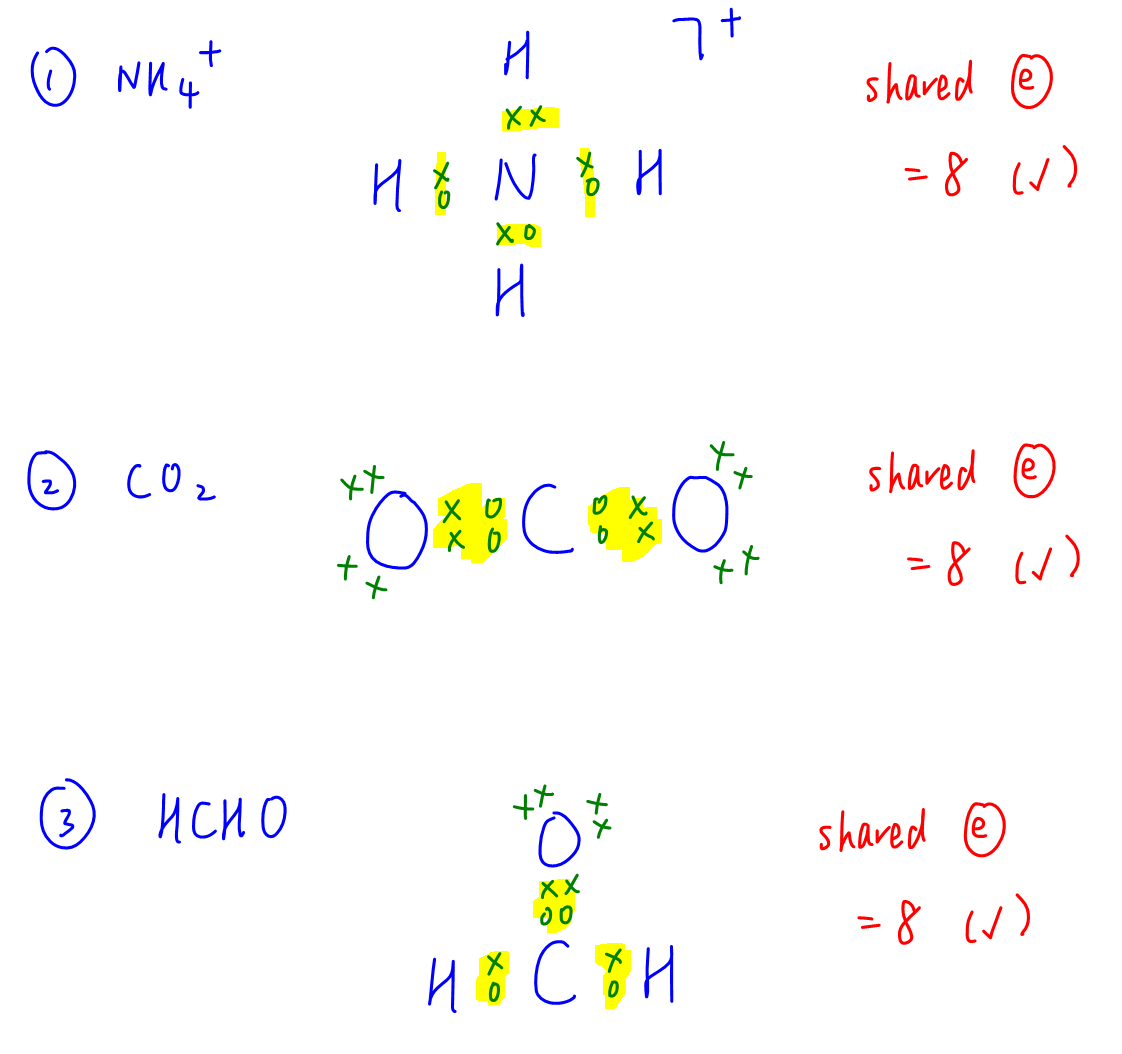
Question 5

Answer: B
Topic: Chemical Bonding
Explanation:
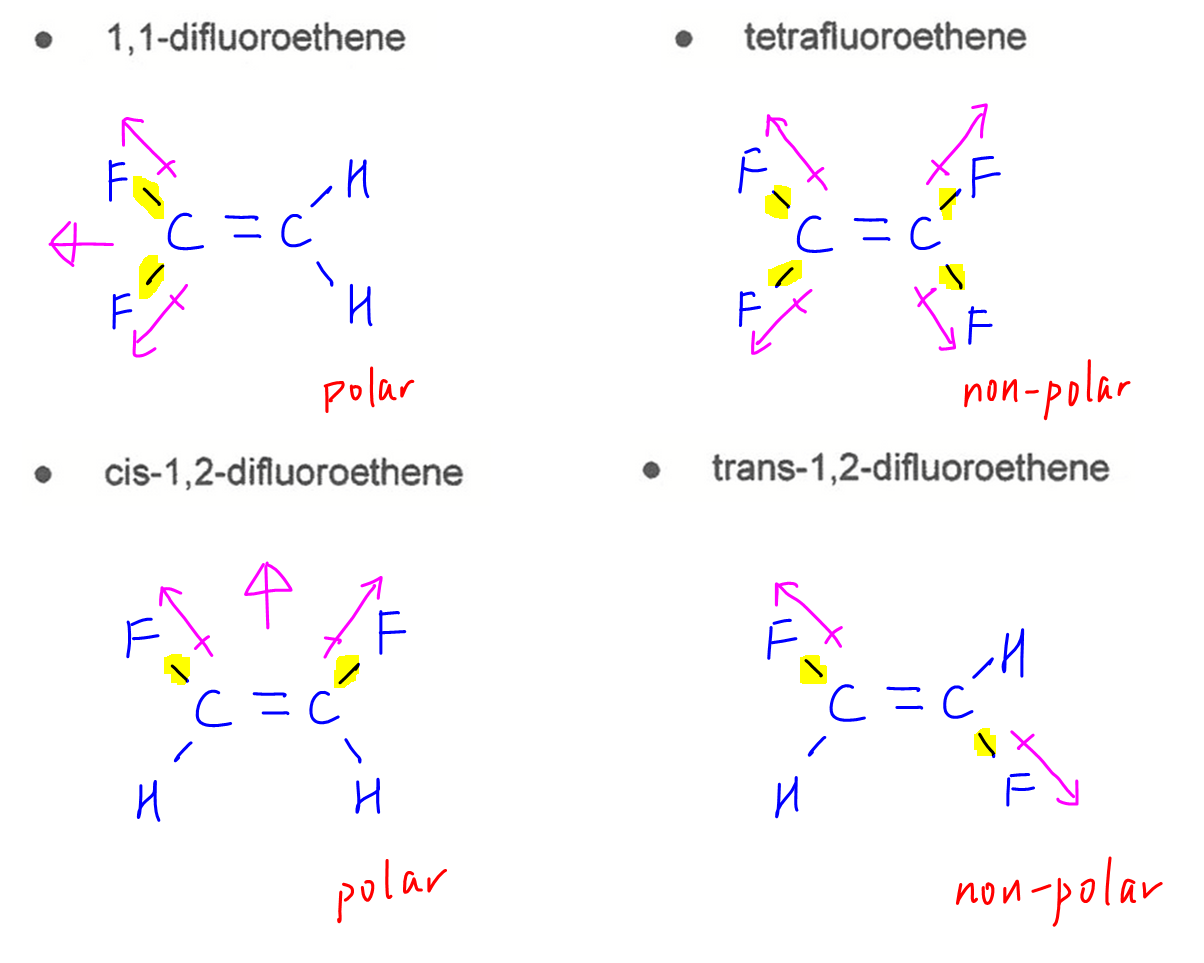
C-F bond is polar and has dipole moment along its bond.
By considering the shape of molecules and their dipole moments due to C-F bonds, we can determine 1,1-difluoroethene and cis-1,2-difluoroethene are polar.
Question 6
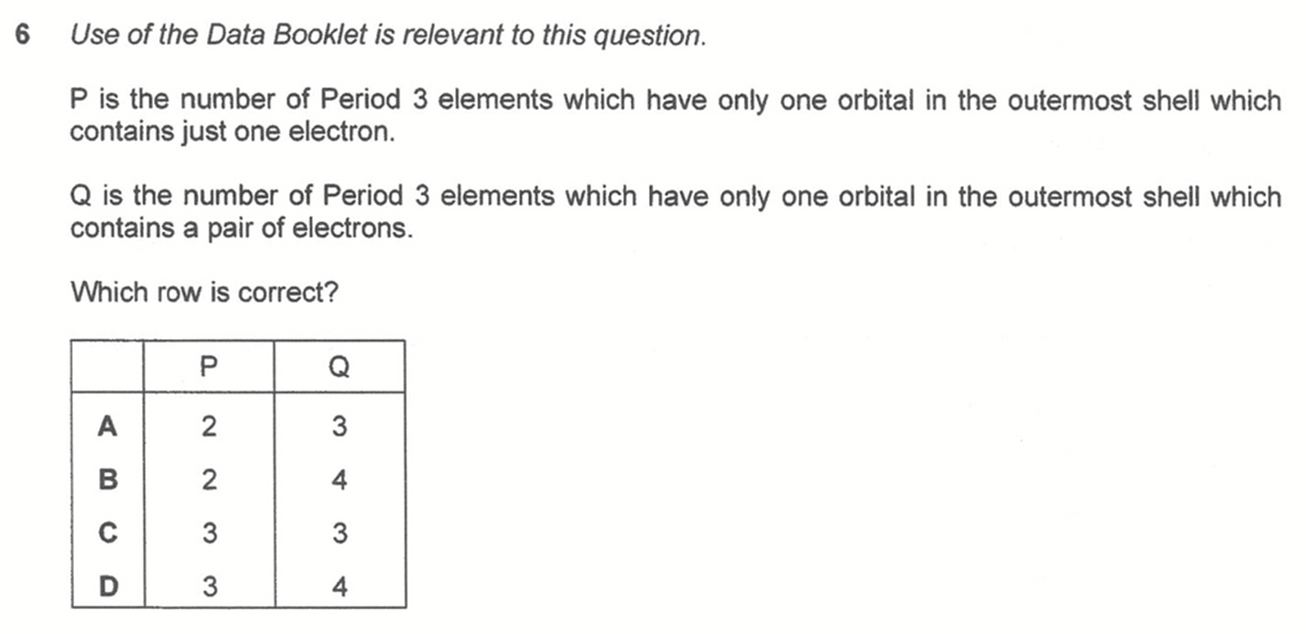
Answer: D
Topic: Atomic Structure
Explanation:
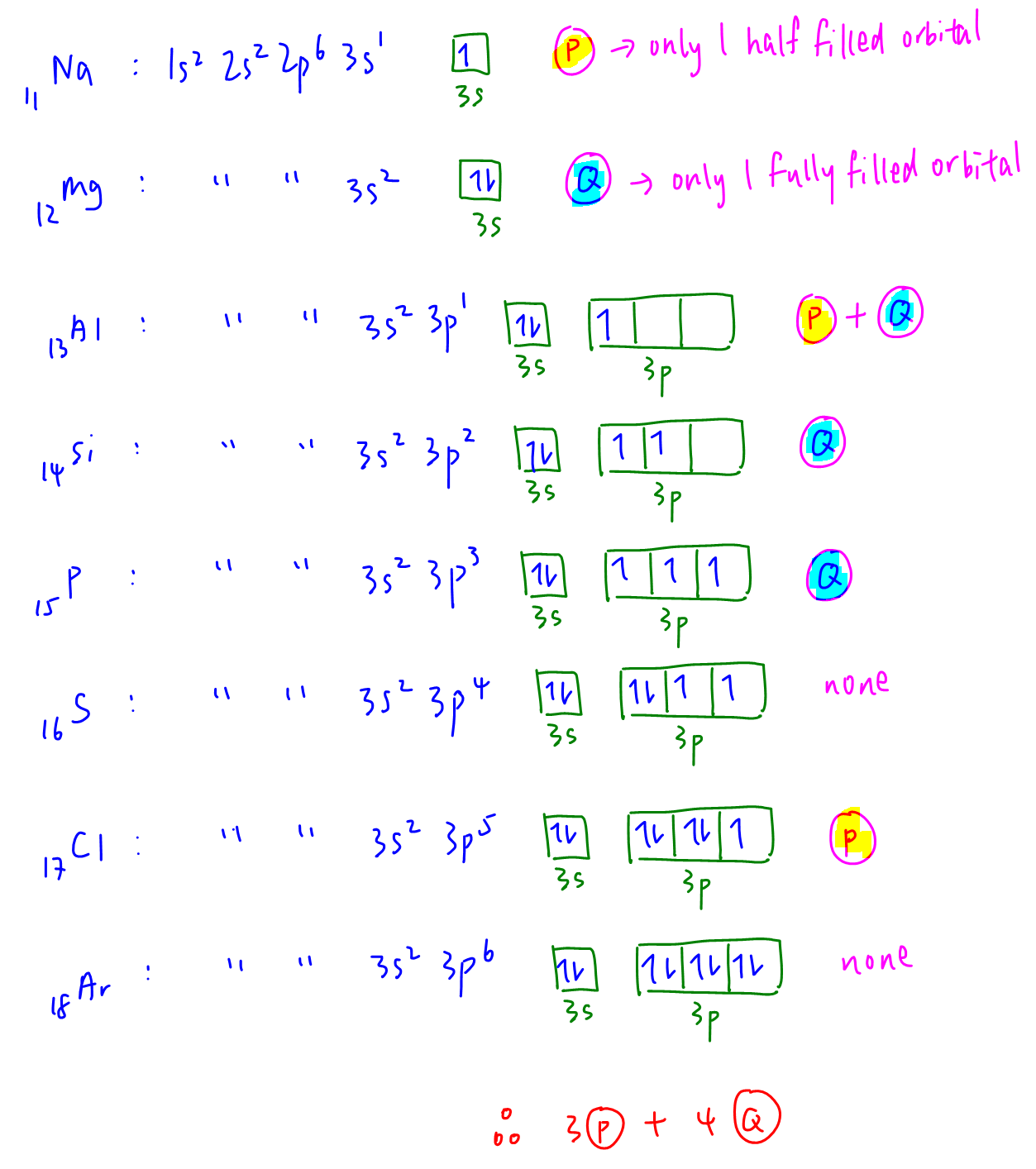
We have to draw out the valence shell electronic configuration via electron in box diagram for Period 3 elements.
From the electronic configurations we can determine that:
P - number of elements with only 1 half filled orbital are Na, Al, Cl (3 elements)
Q - number of elements with only 1 fully filled orbital are Mg, Al, Si, P (4 elements)
Question 7

Answer: A
Topic: Group 2 Elements
Explanation:
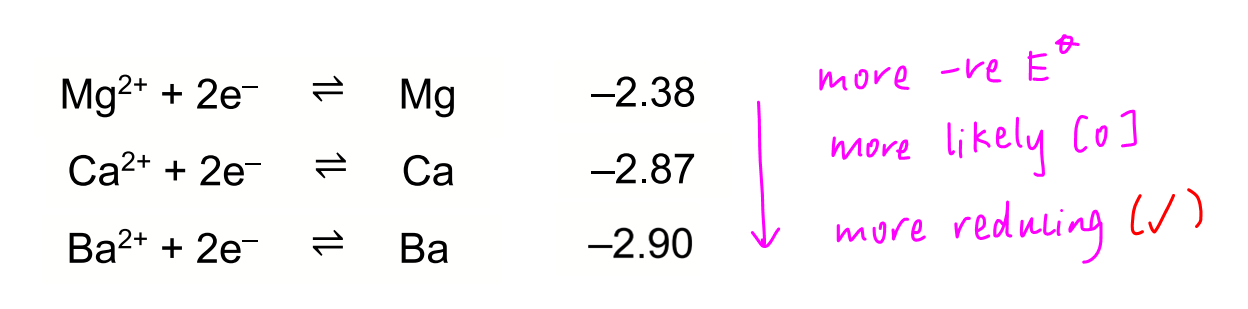
Statement 1 is true as down the group, E value is more negative, metals are more likely oxidised hence more reducing.
Statement 2 is true as down the group, size of metal cation increases and charge density decreases. Metal cations become less polarising and distort electron cloud of carbonate to a smaller extent. The bonds within carbonate are relatively more stable and need more energy to break. Hence thermal stability increases.
Statement 3 is false as charge density of cation should decrease due to increasing size of cation.
Question 8
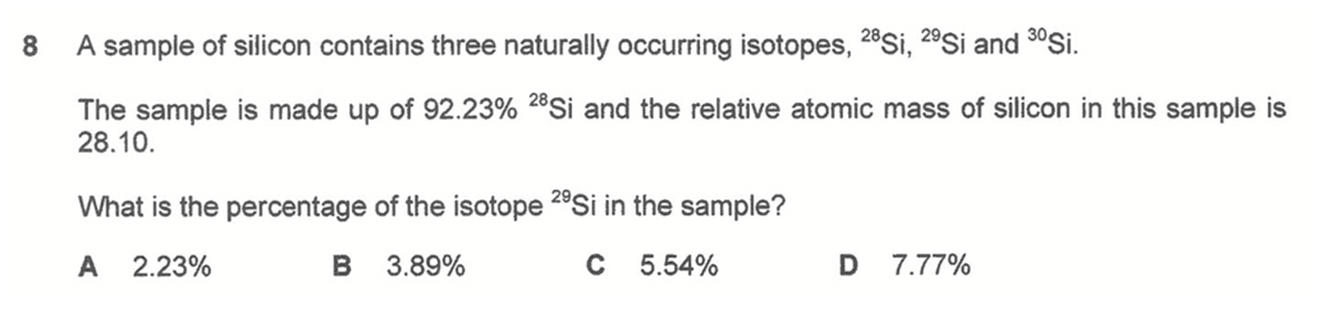
Answer: C
Topic: Atomic Structure
Explanation:
Percentage abundance of 28Si is 92.23%
Let percentage abundance of 29Si be x%, hence percentage abundance of 30Si is (7.77 - x)%
We can then determine relative atomic mass in terms of x and solve for x.
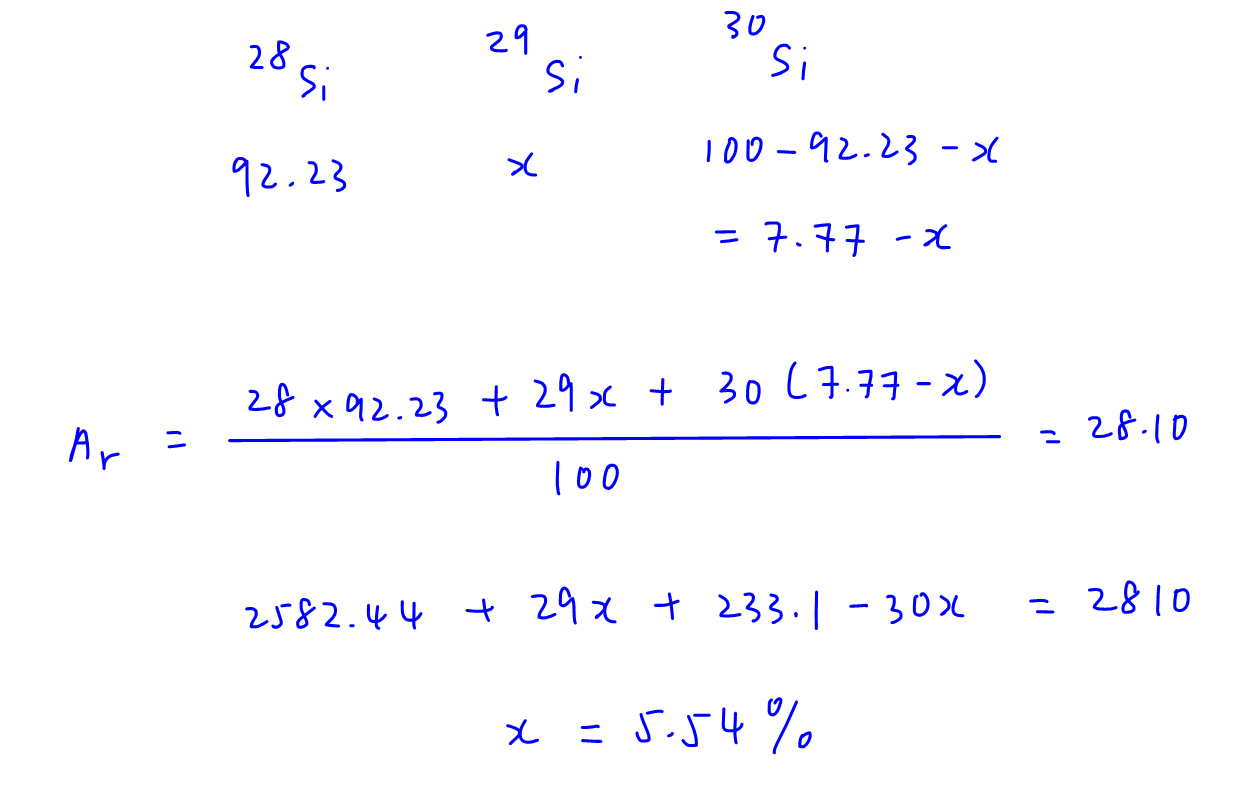
Question 9

Answer: A
Topic: Energetics
Explanation:
NaOH is the limiting reagent so 0.1 mol of NaOH will form 0.1 mol of water.
Heat absorbed by solution = mass of water (150) x c (4.18) x change in temperature (9) = 5.643 kJ = heat released by neutralisation.
Hence enthalpy change of neutralisation can be solved.
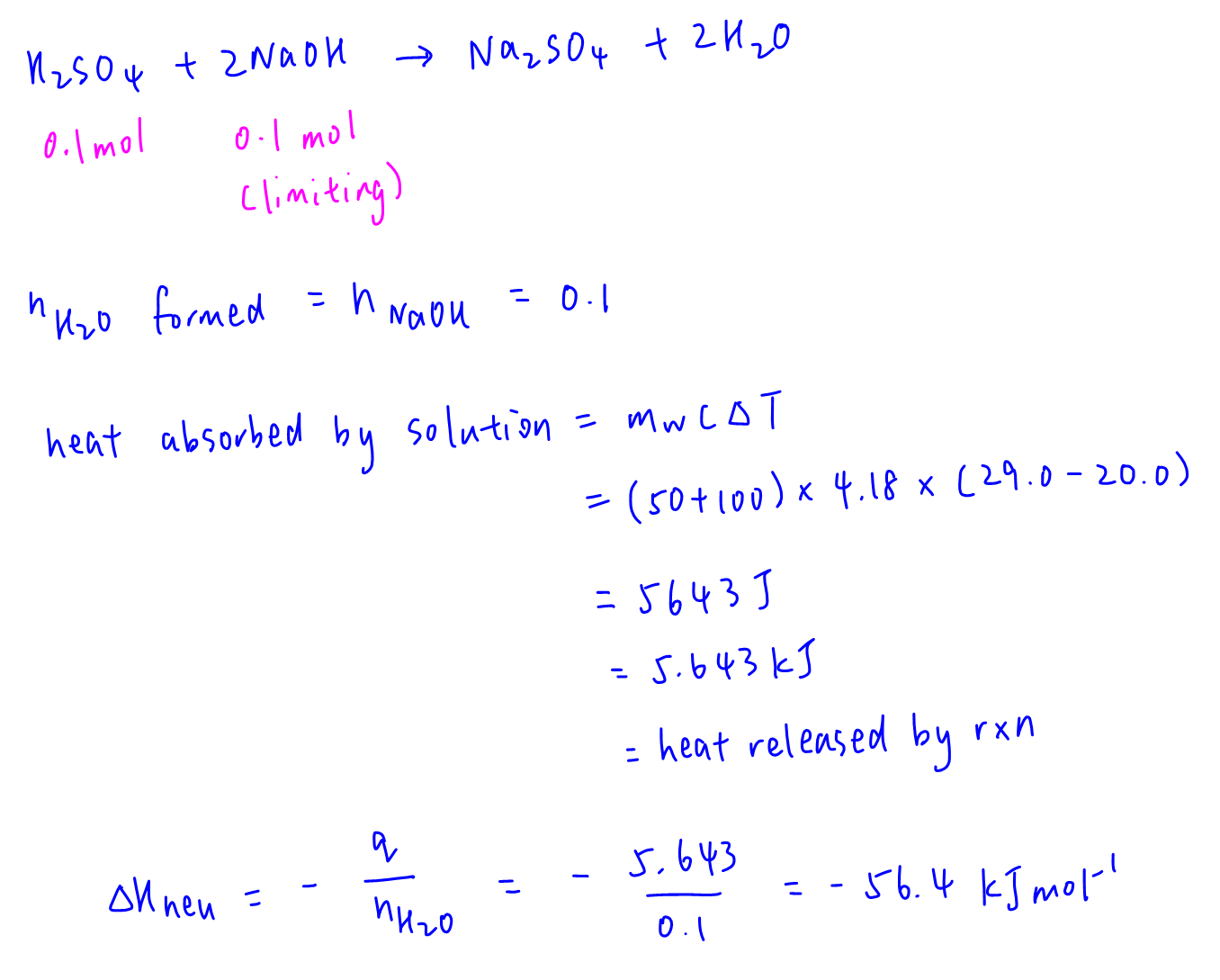
Question 10

Answer: A
Topic: Chemical Bonding
Explanation:
Lattice energy is directly proportionate to charge of cation x charge of anion and inversely proportionate to radius of cation + radius of anion.
Ca+ has smaller charge and bigger size hence lattice energy of CaCl should be the smallest.
Mg2+ has bigger charge and smaller size hence lattice energy of MgCl2 should be the largest.
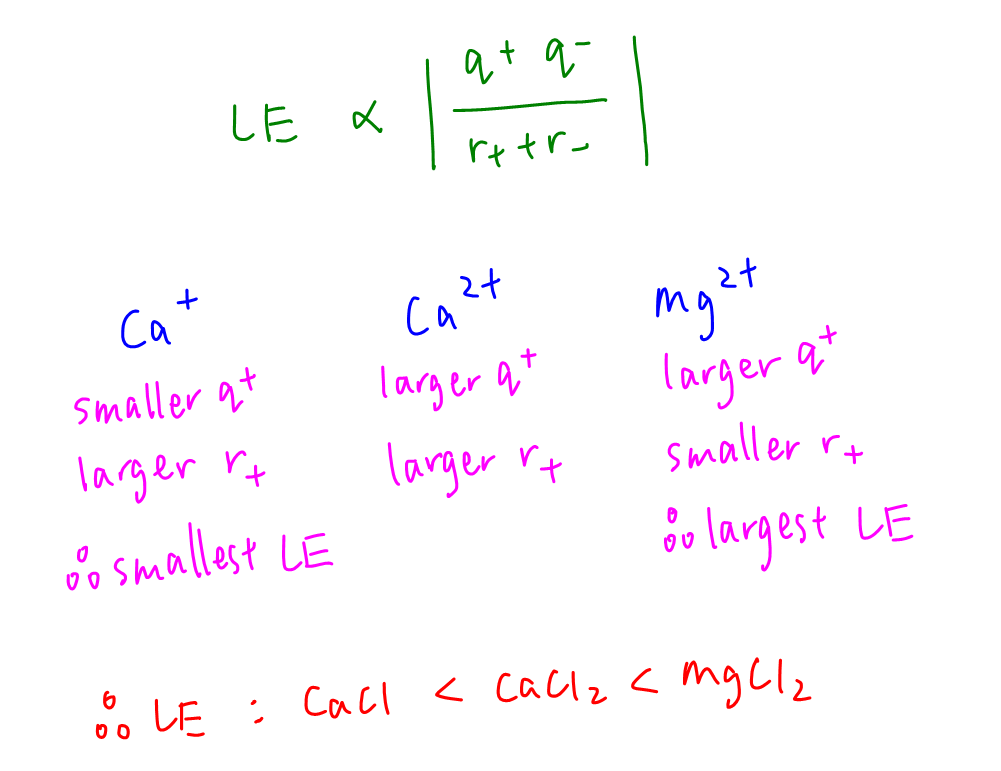
Back to other 2023 A Level Paper 1 Questions
Found this Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new Chemistry videos every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
