2023 A Level H2 Chemistry Paper 1 Solutions - Questions 21 to 30
Question 21

Answer: A
Topic: Halogenoalkane
Explanation:
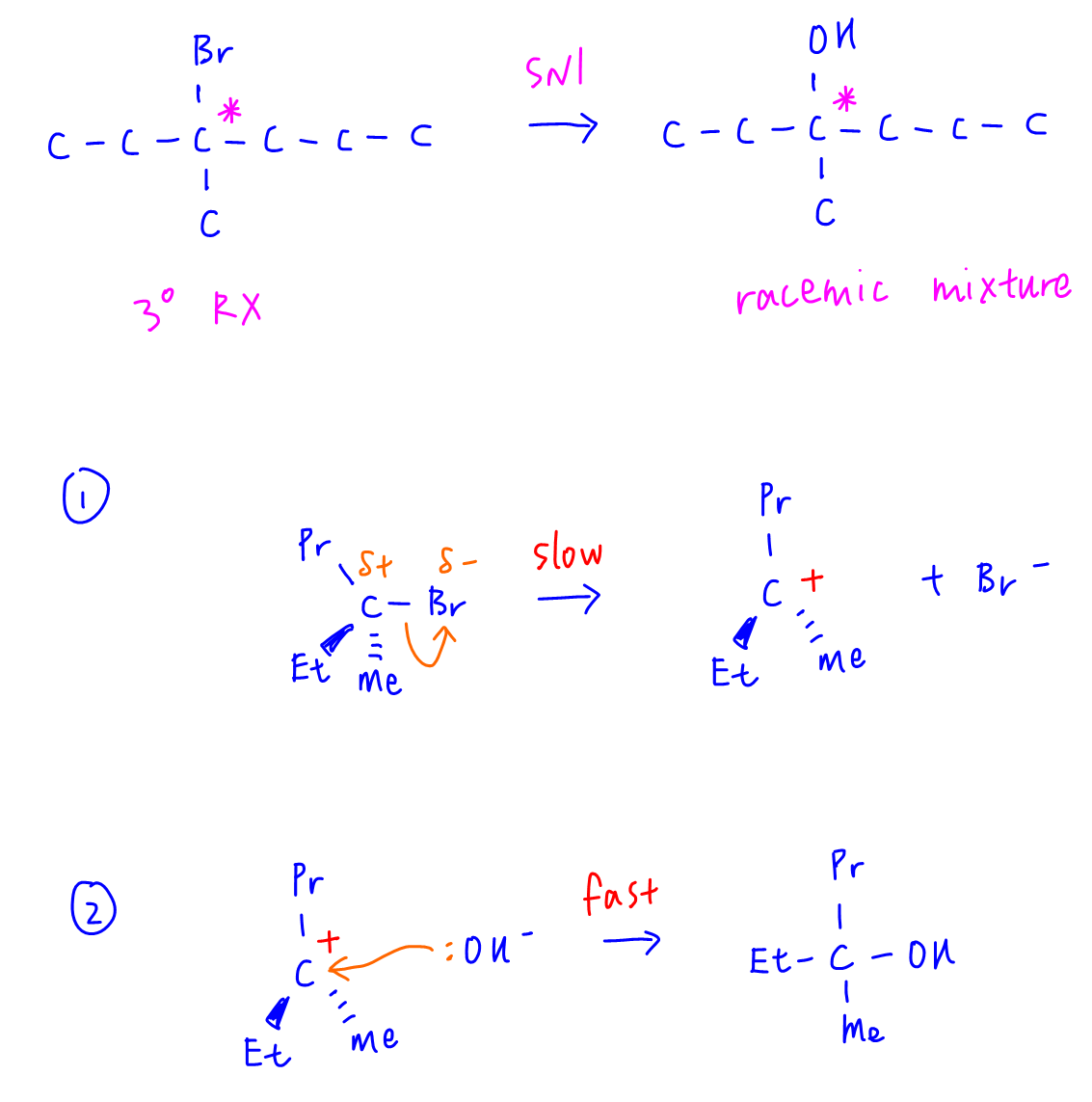
The carbon bonded to bromine in 3-bromo-3-methylhexane is chiral and tertiary. The product mixture of 2 isomeric alcohols indicate mechanism is via SN1 to form an equimolar mixture of 2 optical isomers ie racemic mixture.
Therefore only statement A is consistent with SN1 mechanism.
Question 22

Answer: B
Topic: Halogenoalkane
Explanation:
Comparing hydrolysis reaction, propanoyl chloride is most reactive since acid carbon is bonded to 2 electronegative elements hence acid carbon is most positive and attractive to nucleophiles.
Chlorobenzene is the least reactive as lone pair on Cl is delocalised into pi system of benzene hence C-Cl bond resonance stabilised and unreactive.
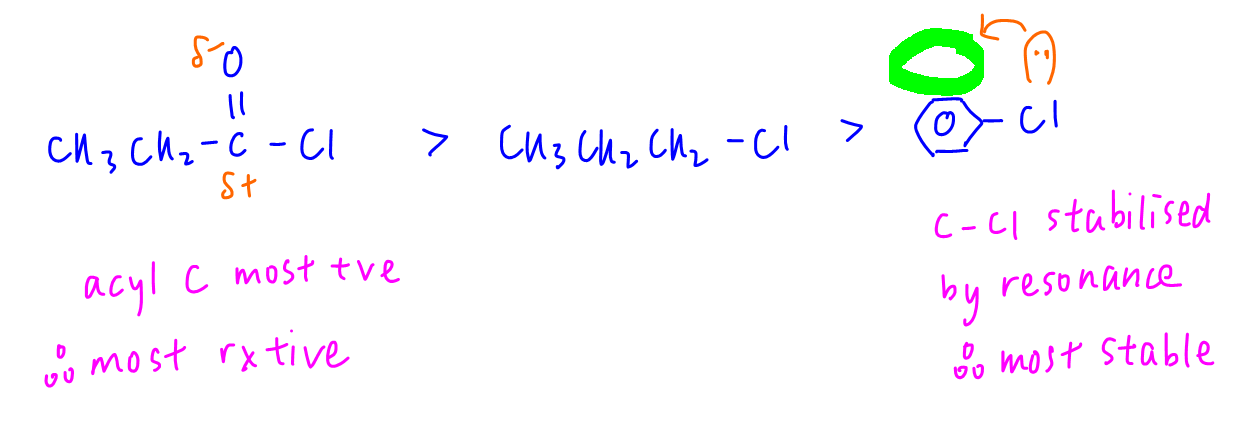
Question 23

Answer: A
Topic: Alkene and Mole Concept
Explanation:
From the balanced equation for combustion of hydrocarbon CxHy, we can write out the mole ratio and volume ratio of CxHy, O2 and CO2.
By comparing mole ratio to volume ratio, we can solve for x = 5 and y = 10. Hence molecular formula for hydrocarbon is C5H10.
C5H10 can be cycloalkane such as unbranched cyclopentane or branched methylcyclobutane. It can also be alkene such as pent-1-ene or pent-2-ene. So all statements are correct.
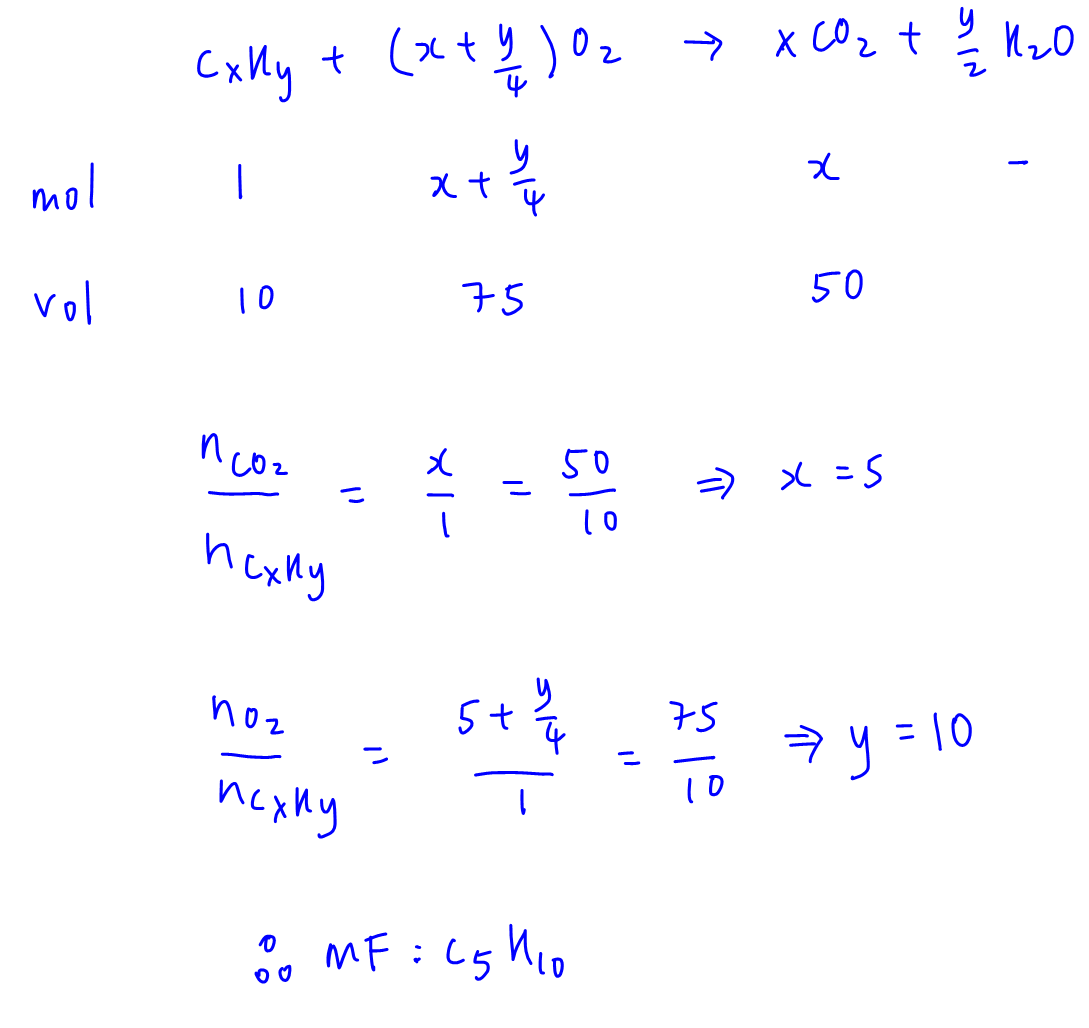
Question 24
Answer: B
Topic: Organic Chem Application
Explanation:
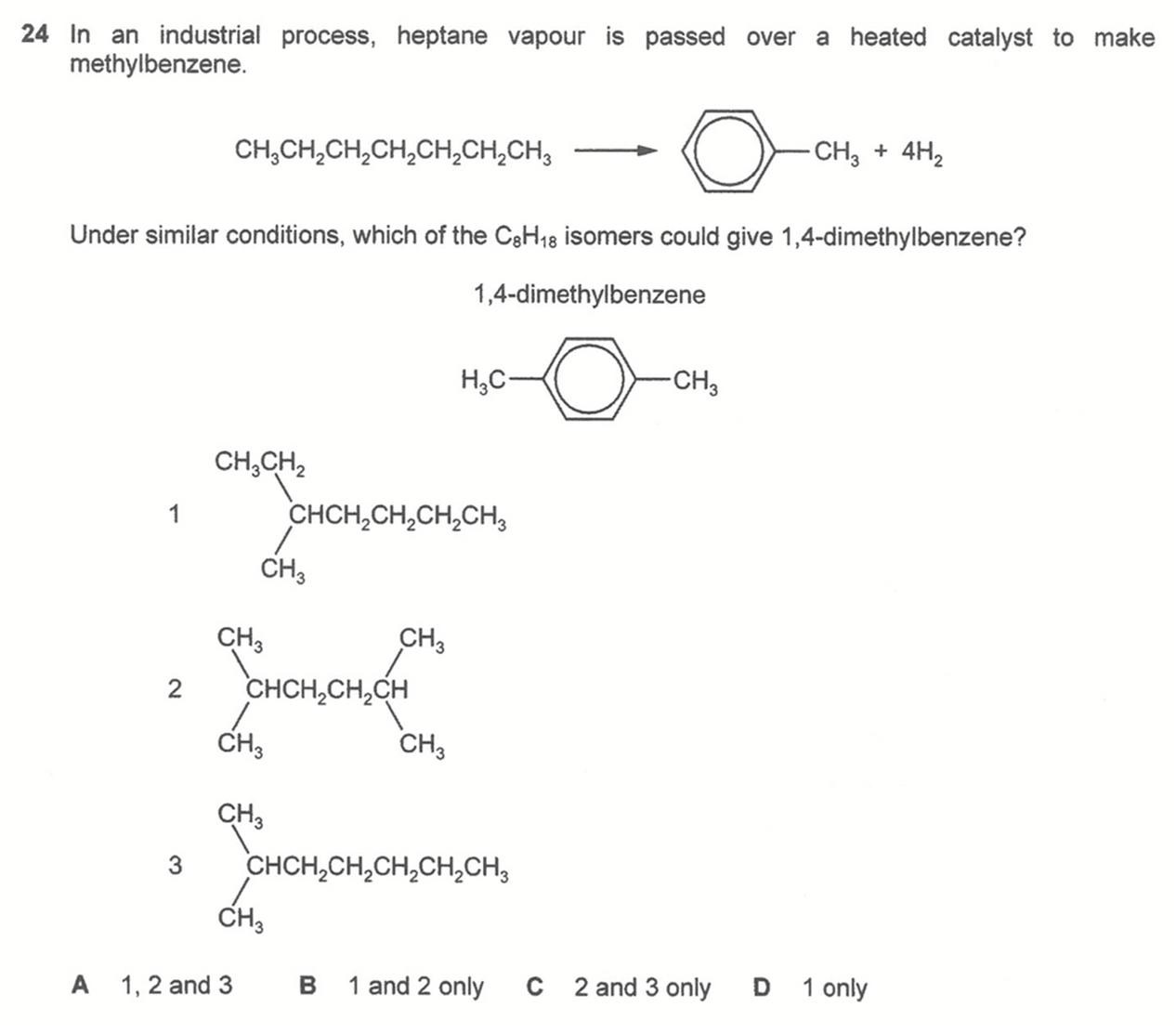
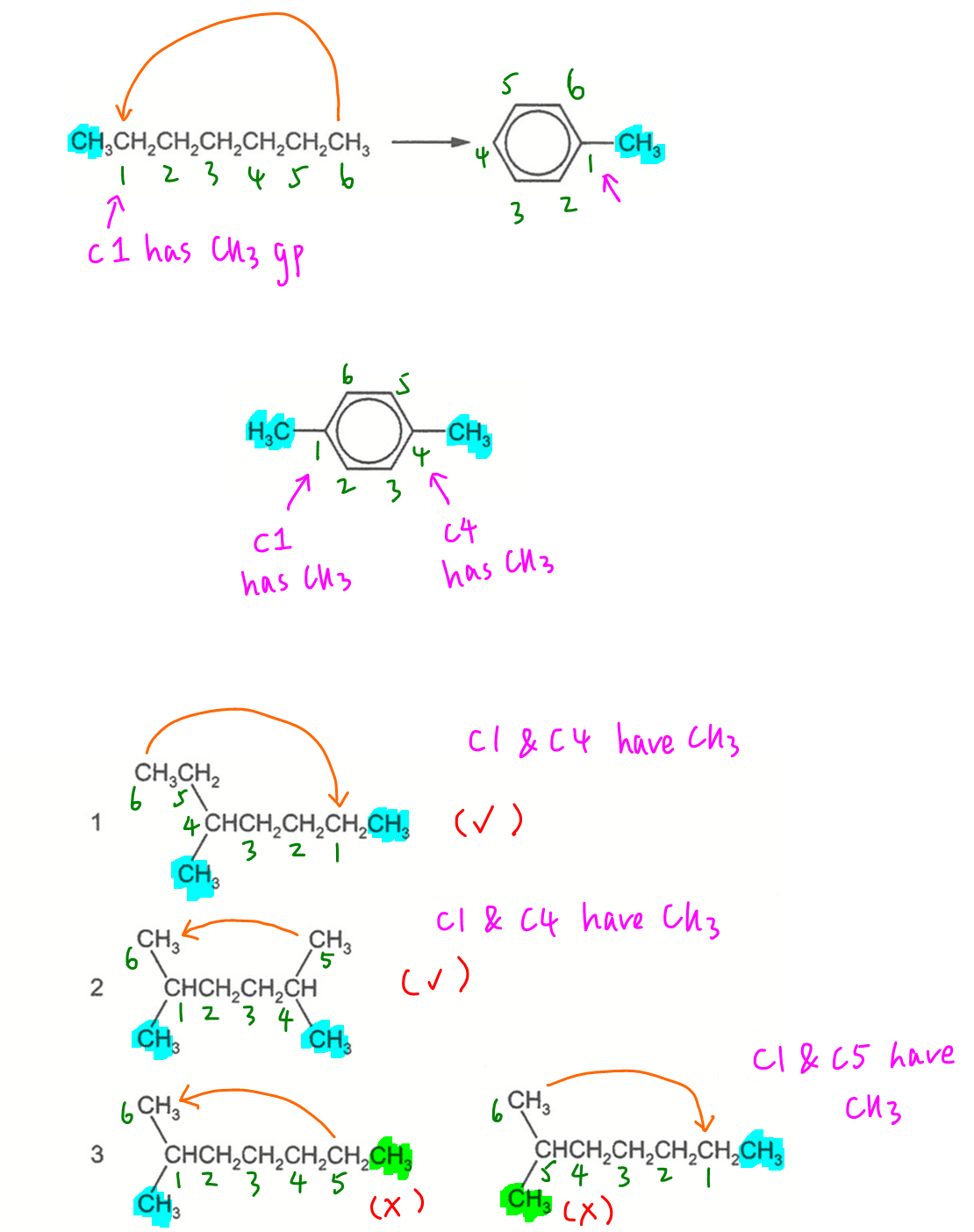
To deduce how heptane can be converted to methylbenzene, we have to draw a closed loop of 6 carbons with a methyl group attached to carbon-1.
Hence in order for the C8H18 isomer to form 1,4-dimethylbenzene, we have to draw a closed loop of 6 carbons with a methyl group attached to carbon-1 and another methyl group attached to carbon-4.
For each of these possible isomers we have to do a bit of trial and error to deduce that isomers 1 and 2 are correct.
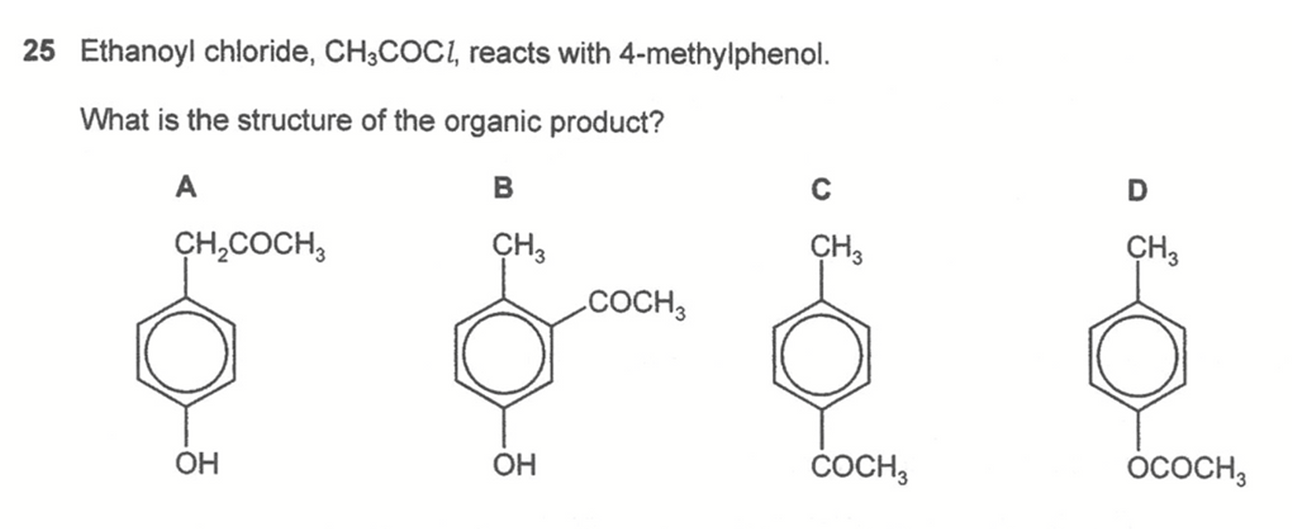
Question 25
Answer: D
Topic: Carboxylic Acid and Derivatives
Explanation:
The reaction is between acid chloride and phenol to form ester functional group. Hence ethanoyl chloride reacts with 4-methylphenol to form 4-methylphenylethanoate.
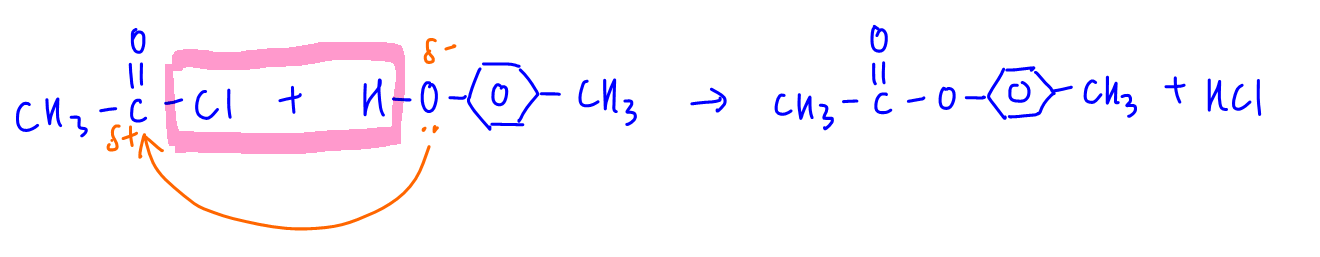
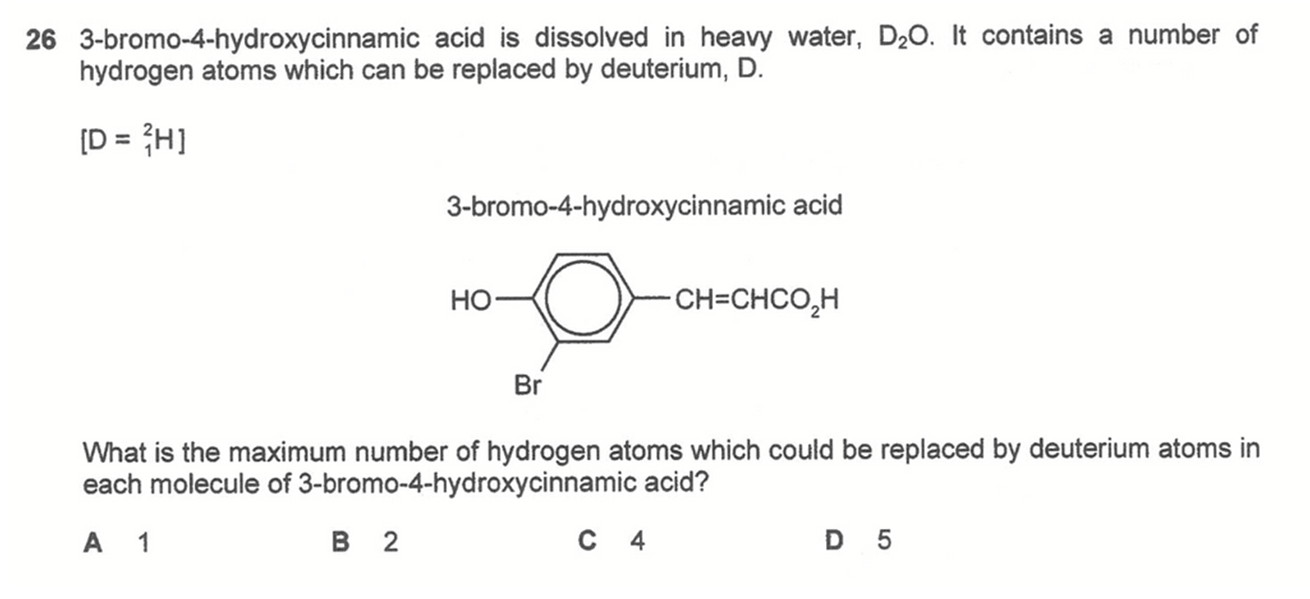
Question 26
Answer: B
Topic: Carboxylic Acid and Derivatives
Explanation:
Functional groups such as carboxylic acids and phenols are more acidic than water and will dissociate in heavy water D2O to form HD2O+ and conjugate base. The reverse process is also possible where conjugate base accepts deuterium cation D+ from HD2O+ hence hydrogen is replaced by deuterium.
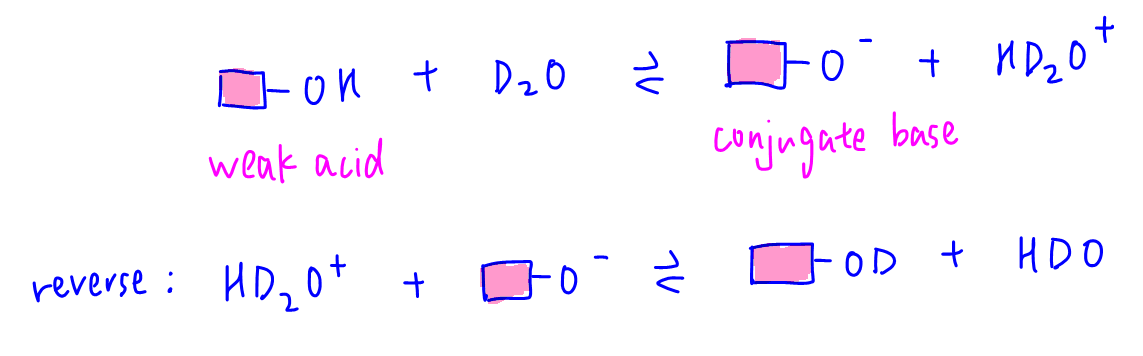
Question 27
Answer: D
Topic: Nitrogen Compounds and Proteins
Explanation:
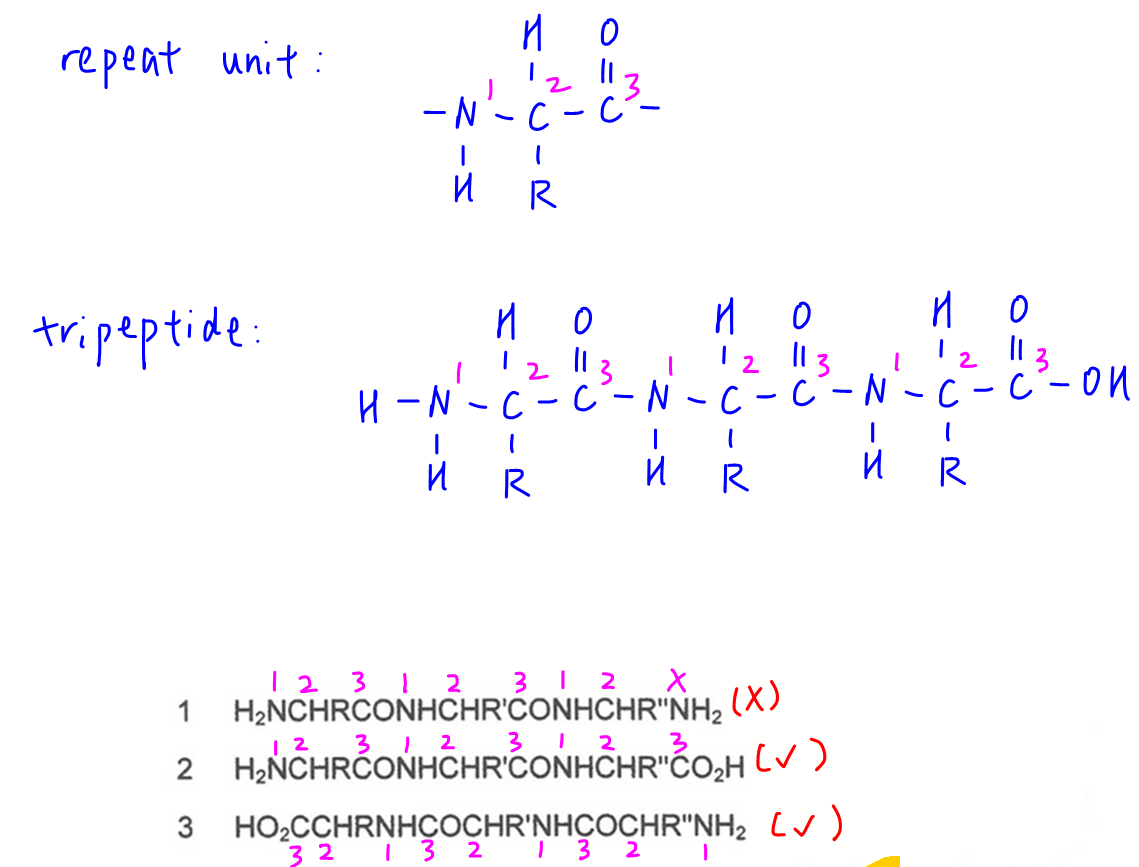
The repeat unit of primary structure of protein is -NHCHRCO- with positions:
1 - amine N
2 - carbon with R group
3 - acid C
Hence a tripeptide will also have the same repeat unit thrice.
Only structures 2 and 3 follow this repeat unit and are tripeptides.
Question 28

Answer: A
Topic: Electrochemistry
Explanation:
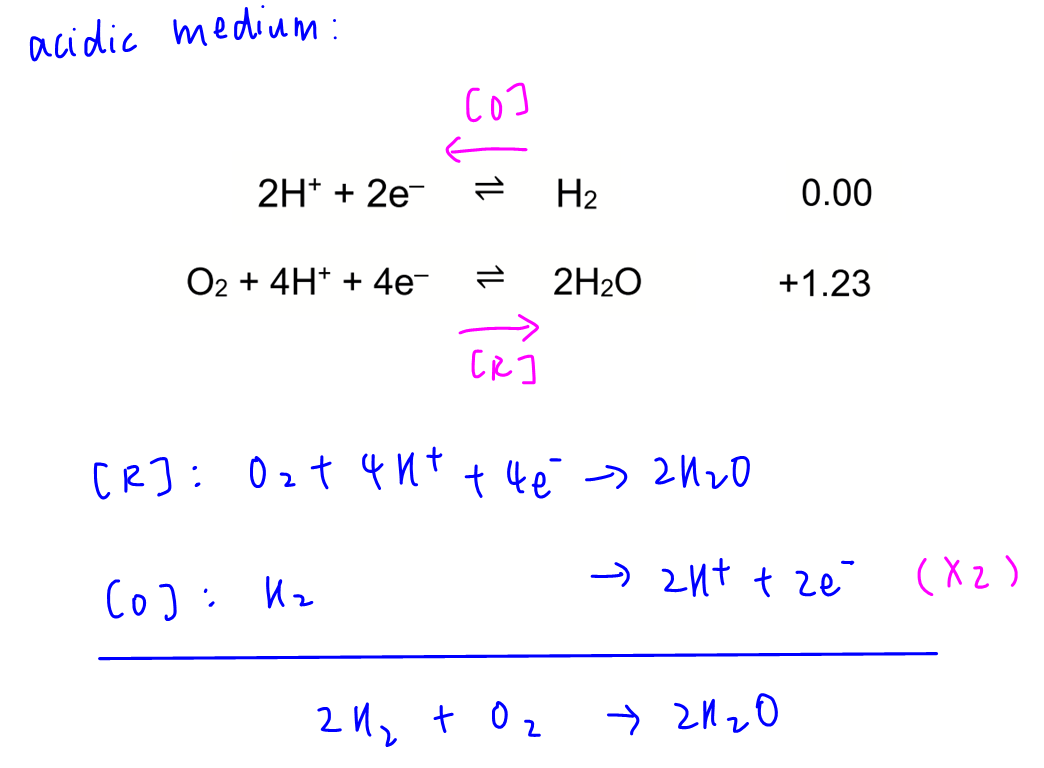
Statement 1 is correct since only water is formed from hydrogen-oxygen fuel cell.
Statement 2 is correct.
Statement 3 is correct as hydrogen is oxidised to H+ hence electrons are released.
Question 29

Answer: B
Topic: Electrochemistry
Explanation:
Statement 1 is correct. When standard hydrogen electrode is attached to a half-cell with negative E, SHE will have more positive E and undergoes reduction to form H2 gas.
Statement 2 is wrong since standard redox potential for SHE is 0.00V.
Statement 3 is wrong as 1.00 moldm-3 H2SO4 will give 2.00 moldm-3 H+ which is not standard conditions of 1.00 moldm-3.
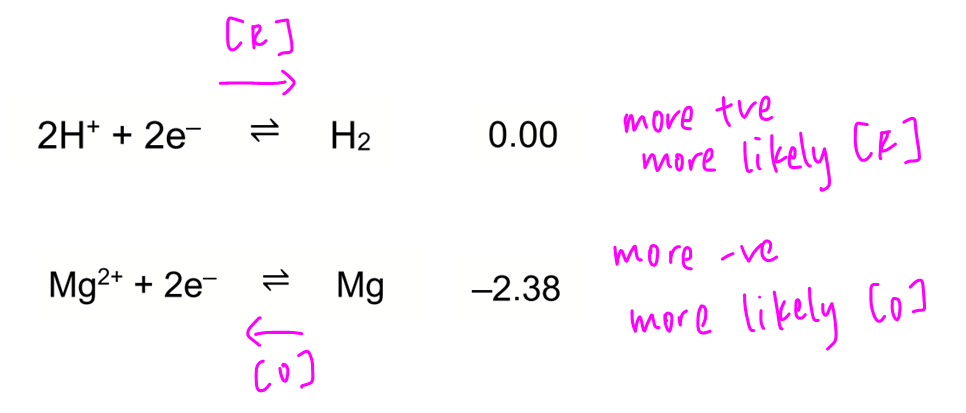
Question 30
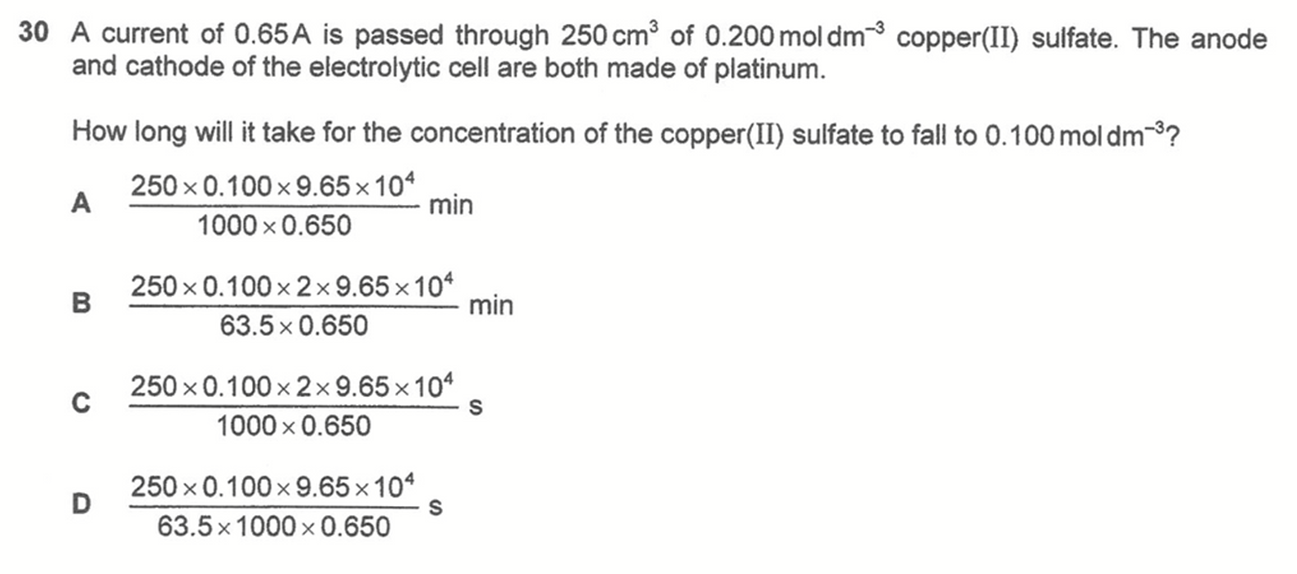
Answer: C
Topic: Electrochemistry
Explanation:
From decrease in concentration of Cu2+ we can calculate moles of Cu2+ reduced and moles of electrons transferred.
Then we can use Faraday's equations to write an expression involving time t.
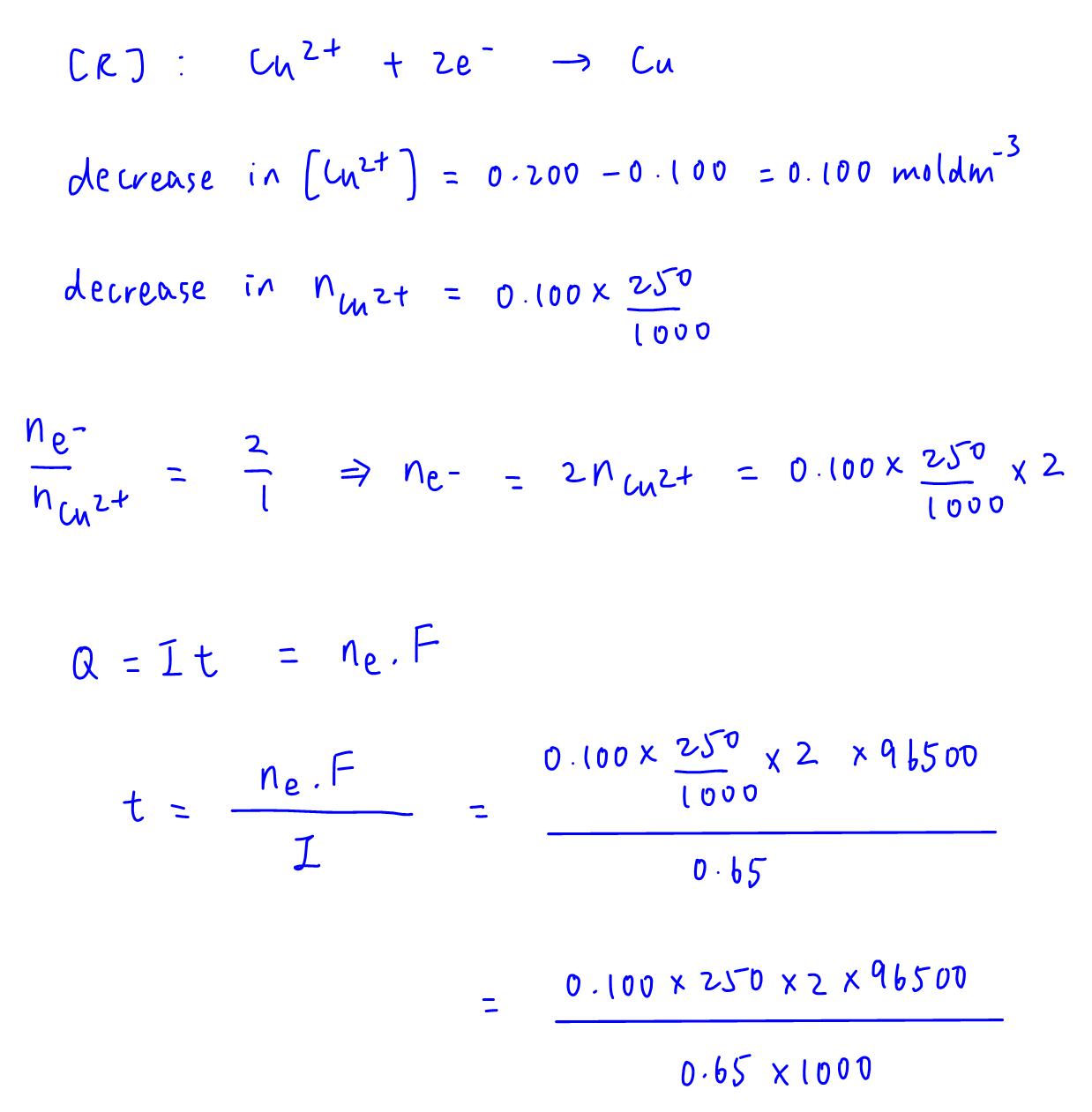
Back to other 2023 A Level Paper 1 Questions
Found this Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new Chemistry videos every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
