2024 A Level H2 Chemistry Paper 1 Solutions - Questions 11 to 20
Question 11
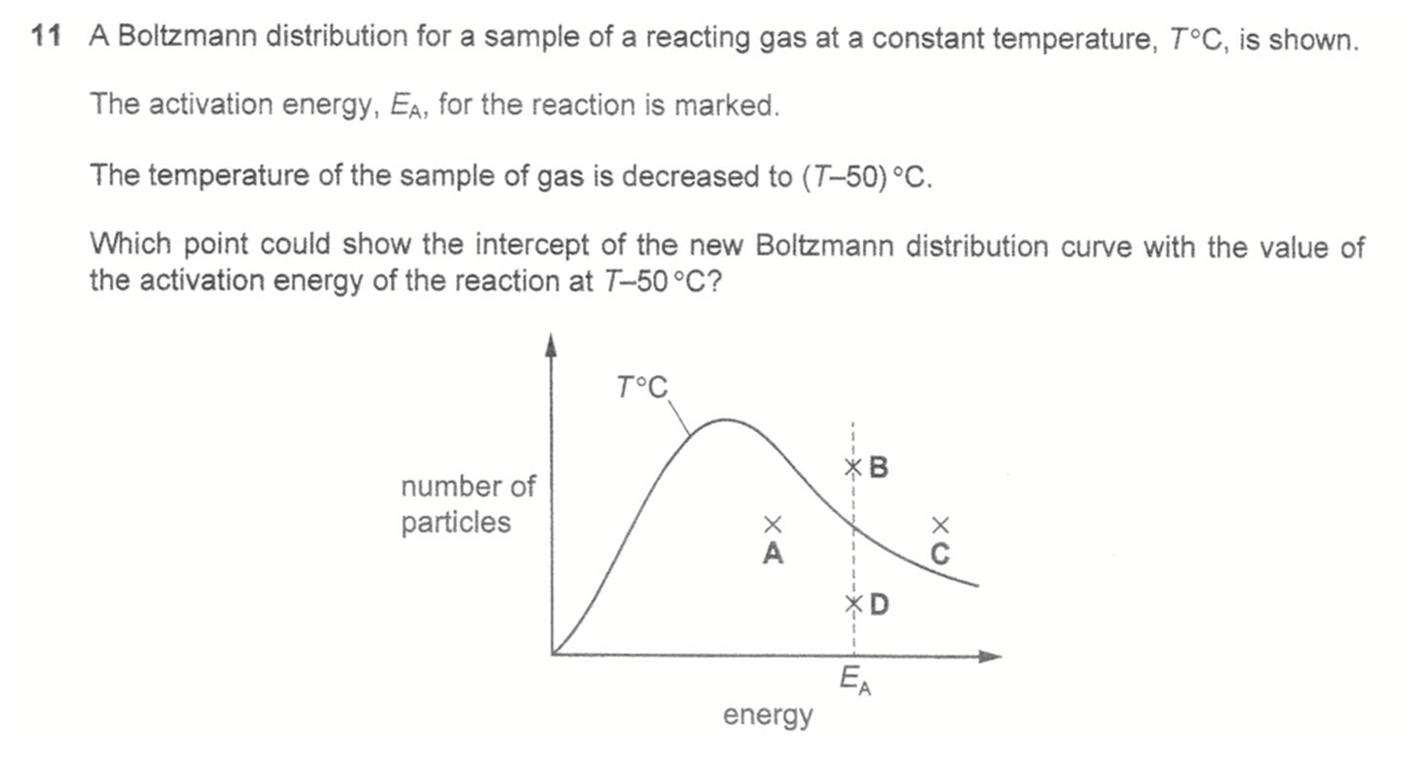
Answer: D
Topic: Kinetics
Explanation:
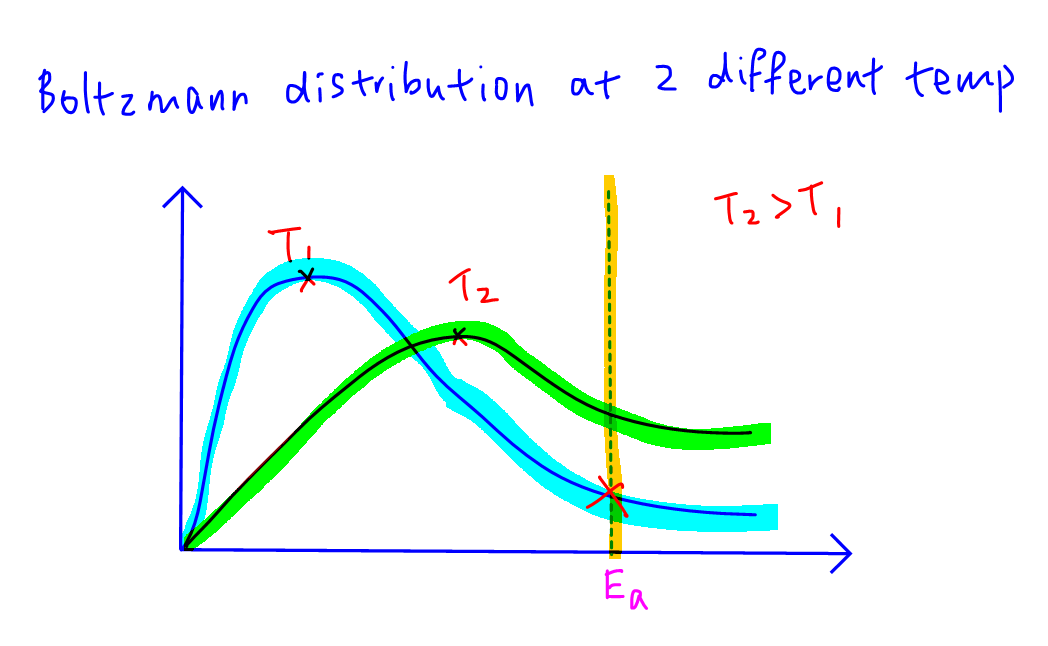
At a lower temperature the Boltzmann distribution curve will shift left and intercept with the activation energy at a lower point.
Question 12
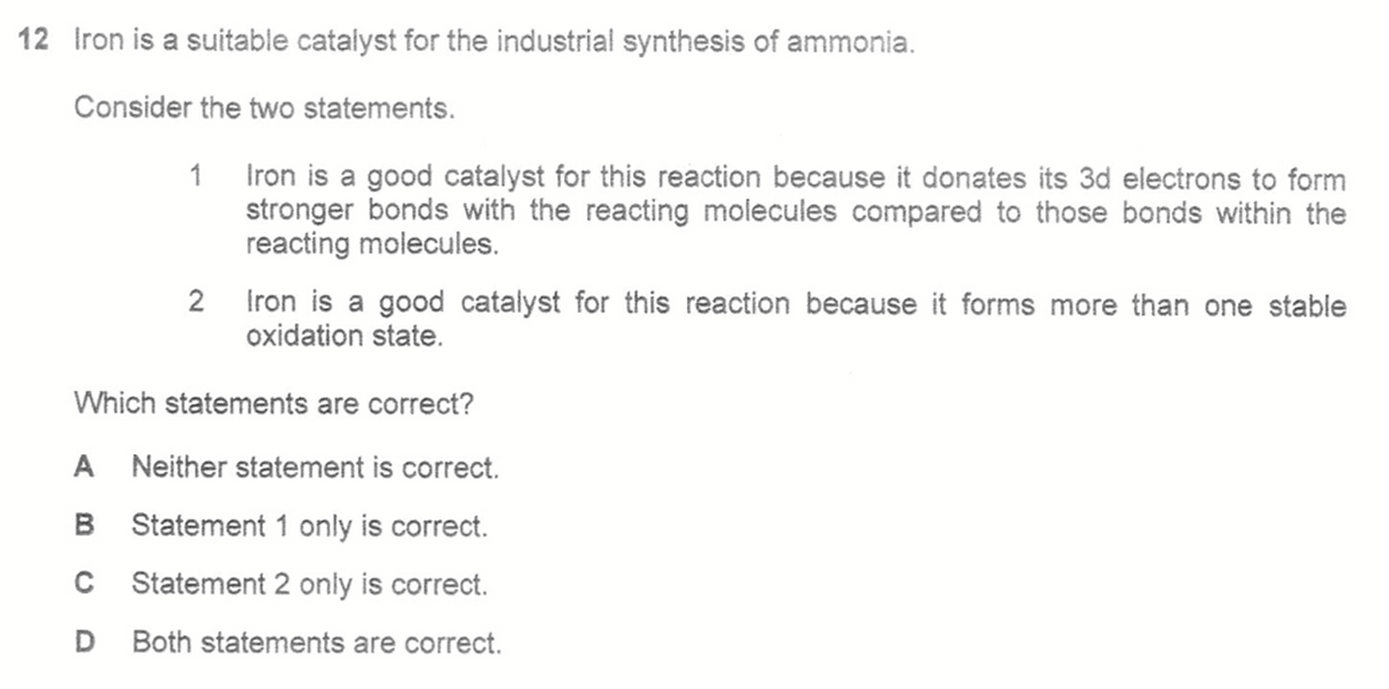
Answer: A
Topic: Chemical Equilibria, Transition Elements
Explanation:
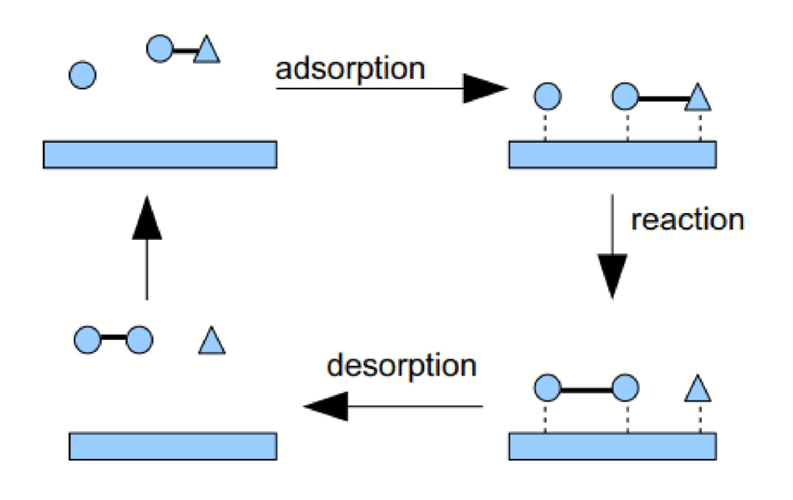
Iron acts as a heterogeneous catalyst in Haber Process. The gaseous reactants adsorb onto the surface of the metal by overlapping with the d-orbitals of iron atoms which results in the following:
1. bonds within reactants weaken
2. concentration of reactants on metal surface increases
Hence rate of reaction on metal surface increases which speeds up the overall reaction.
Therefore statement 1 is false since the process of adsorption does not involve donation of 3d electrons.
Statement 2 is false since iron does not undergo change in oxidation state when acting as a heterogeneous catalyst.
Question 13

Answer: C
Topic: Chemical Equilibria
Explanation:
We can let the change in moles of reactants be x and fill up the ICE table in terms of x.
We can then substitute the equilibrium concentrations of reactants and products into the known equilibrium constant and solve for x and concentration of H2 at equilibrium.
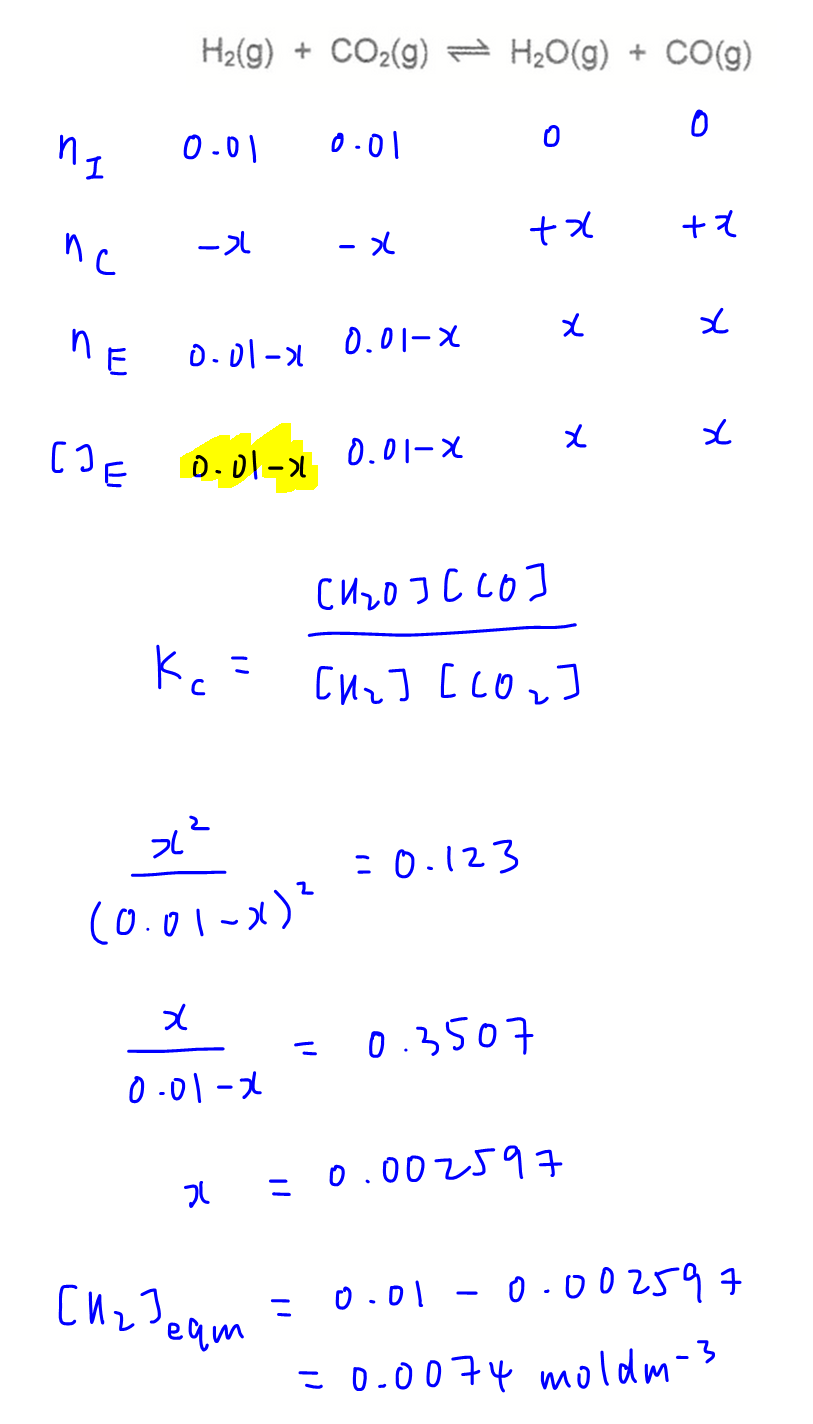
Question 14
Answer: B
Topic: Periodicity
Explanation:
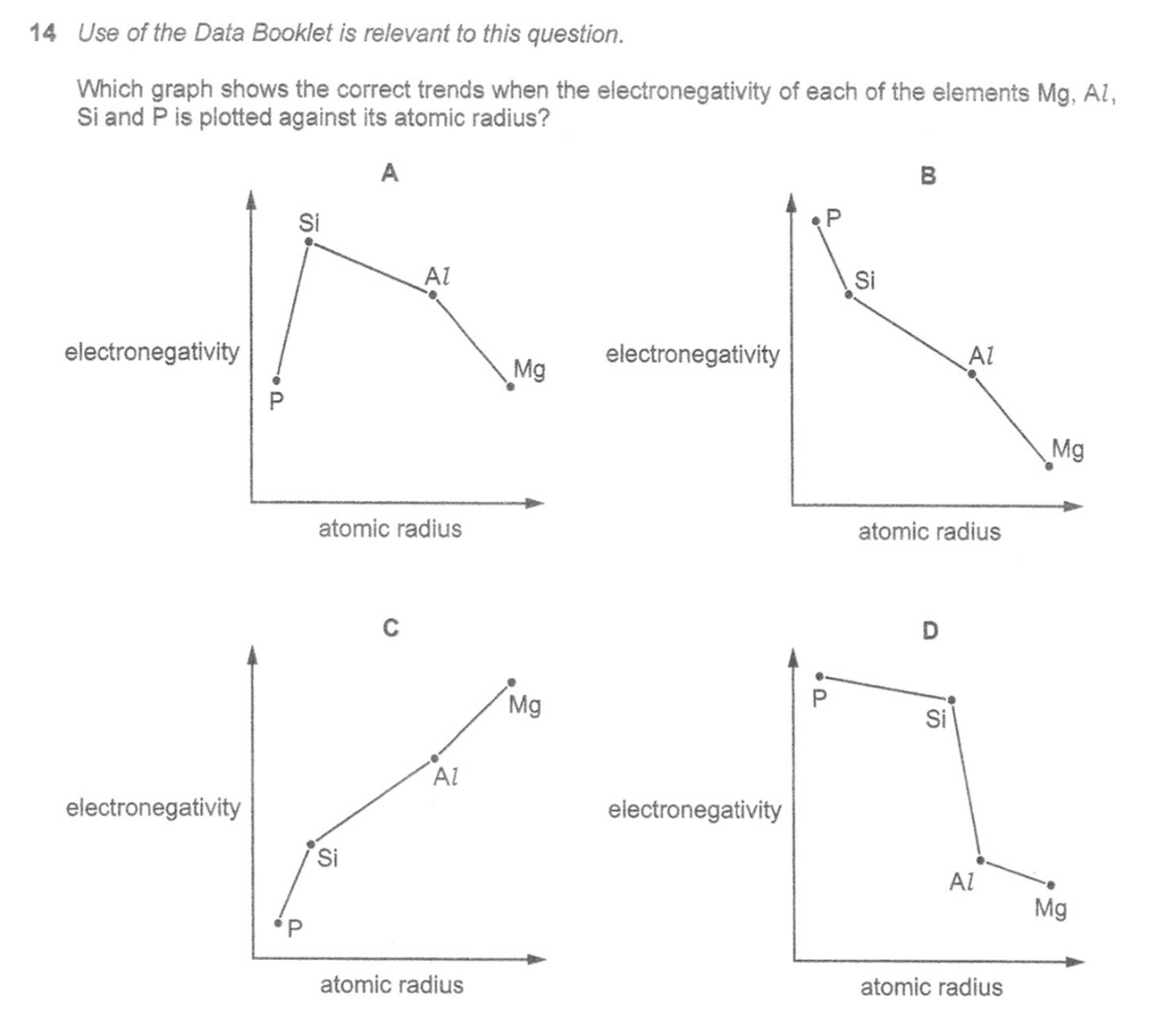
Electronegativity increases across the period in the order: Mg (least electronegative), Al, Si, P (most electronegative).
Atomic radius decrease across the period in the order: P (smallest), Si, Al, Mg (largest). From the data booklet we can find the biggest jump in atomic radius from Si to Al.

Question 15

Answer: A
Topic: Redox Reactions
Explanation:
Metals are the reducing agents since they are oxidised while non-metals are the oxidising agents since they are reduced.
From the oxidation states of the elements in the compounds we can deduce which option is the change in oxidation state for metal (reducing agent) greater than that for non-metal (oxidising agent).

Question 16
Answer: D
Topic: Buffer and Titration Curve
Explanation:
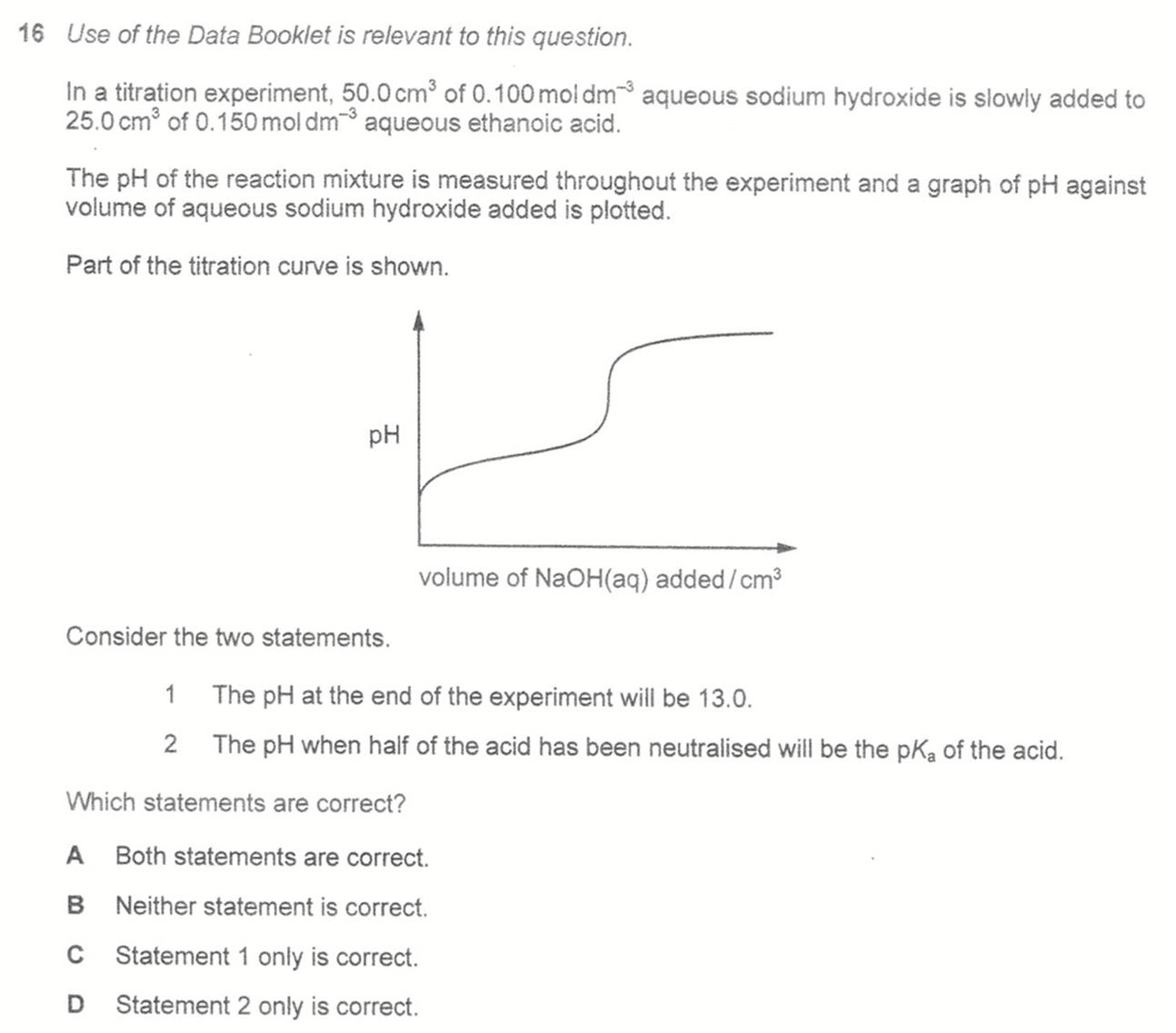
Statement 1 is false. At the end of the experiment there is excess NaOH. We can calculate the amount of remaining NaOH, its concentration and hence its pH to be 12.2, not 13.0.

Statement 2 is true. When half of the acid has been neutralised (half equivalence), the resultant solution is a buffer at maximum buffering capacity hence pH = pKa.

Question 17

Answer: C
Topic: Buffer and Titration Curve
Explanation:
We can use the ICE table to determine the resultant solution. Since the solution is a buffer, we can use the buffer equation to calculate its pH.
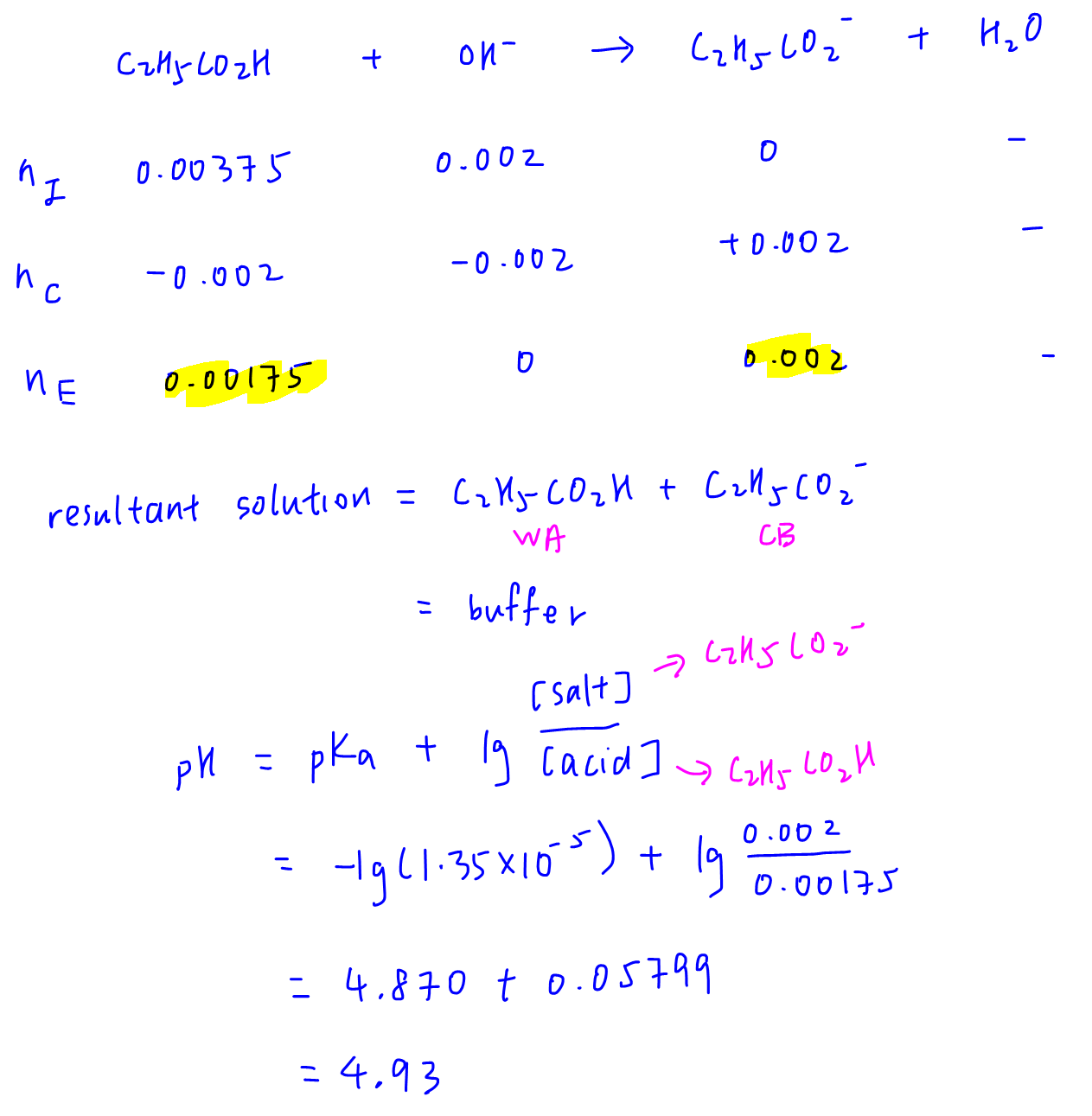
Question 18

Answer: D
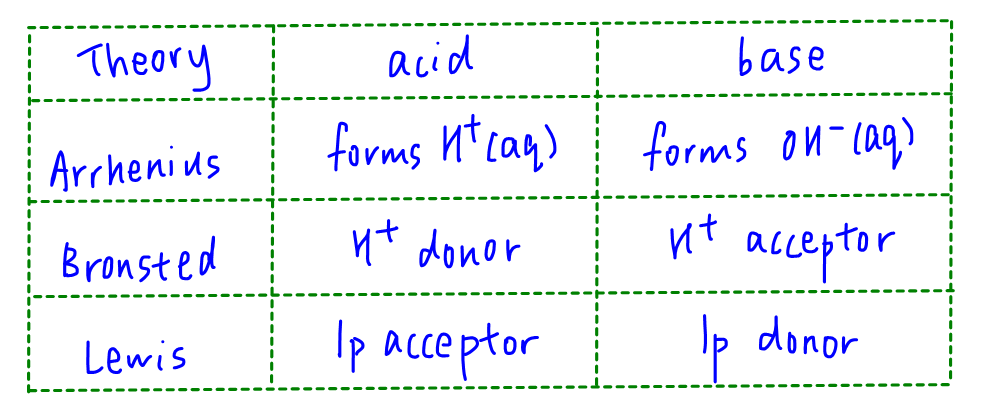
Topic: Carbonyl Compounds, Acid-Base Theory
Explanation:

During Grignard reaction the CH3CH2- acts as nucleophile to attack carbonyl carbon which is partial positive charge. Since CH3CH2- is electron rich with a lone pair to donate, it behaves as Lewis bases.
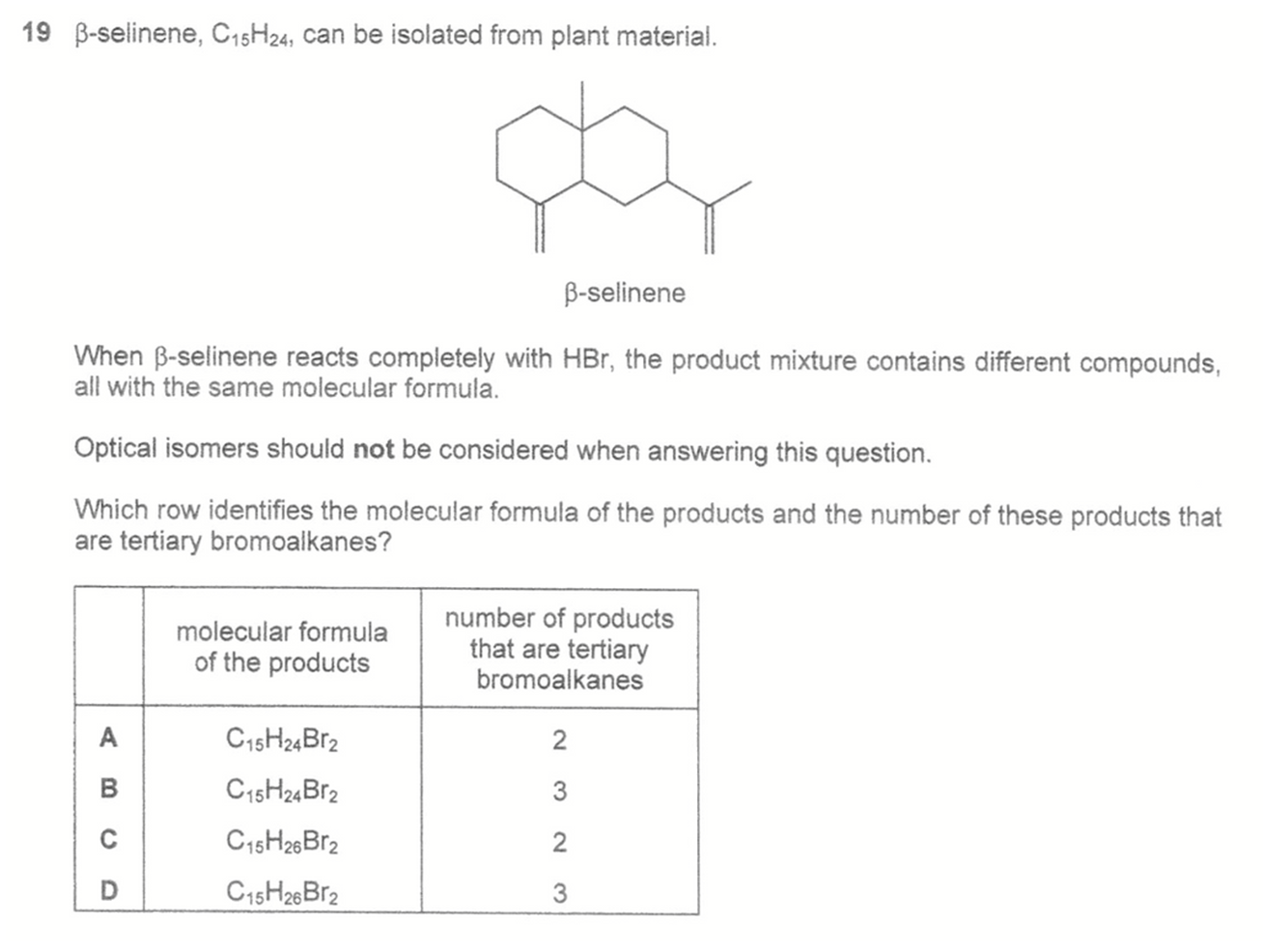
Question 19
Answer: D
Topic: Alkenes
Explanation:
Alkene with HBr is an addition reaction.
1. Determine molecular formula
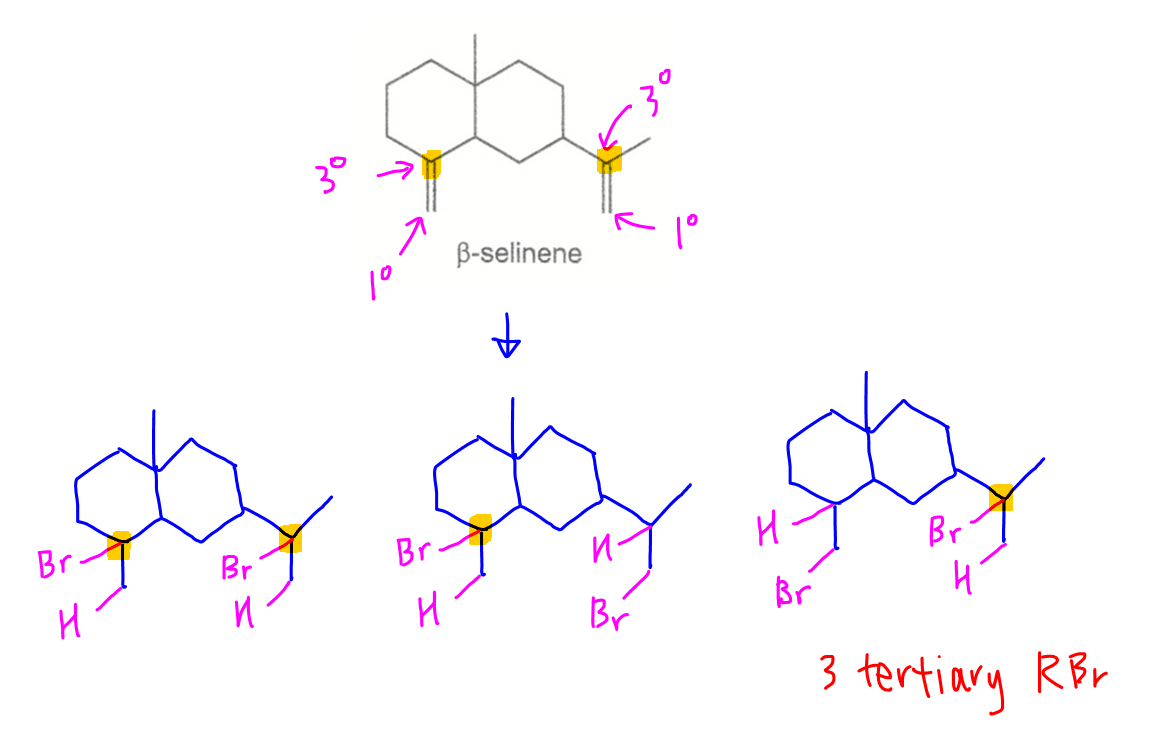
Selinene (C15H24) has 2 alkene functional groups so will add 2 HBr to form C15H26Br2.
2. Deduce tertiary bromoalkanes
There are 2 tertiary alkene carbons that bromine can be added to form tertiary bromoalkanes. Hence there are 3 possible products that have at least 1 tertiary bromoalkane.
Question 20

Answer: B
Topic: Alkenes
Explanation:
When HBr is added to propene, 2-bromopropane is the major product according to Markovnikov rule (H joins to C with more H).
We can explain this using stability of carbocations.
In the first step of electrophilic addition mechanism, electrophile H can be added to 2 different alkene carbons to form 2 possible carbocations.
When H is added to the alkene carbon with more H, it forms a more substituted carbocation with more electron donating methyl groups that disperse positive charge on carbocation and stabilises it.
This makes that carbocation more likely formed and hence gives us the major product 2-bromopropane.
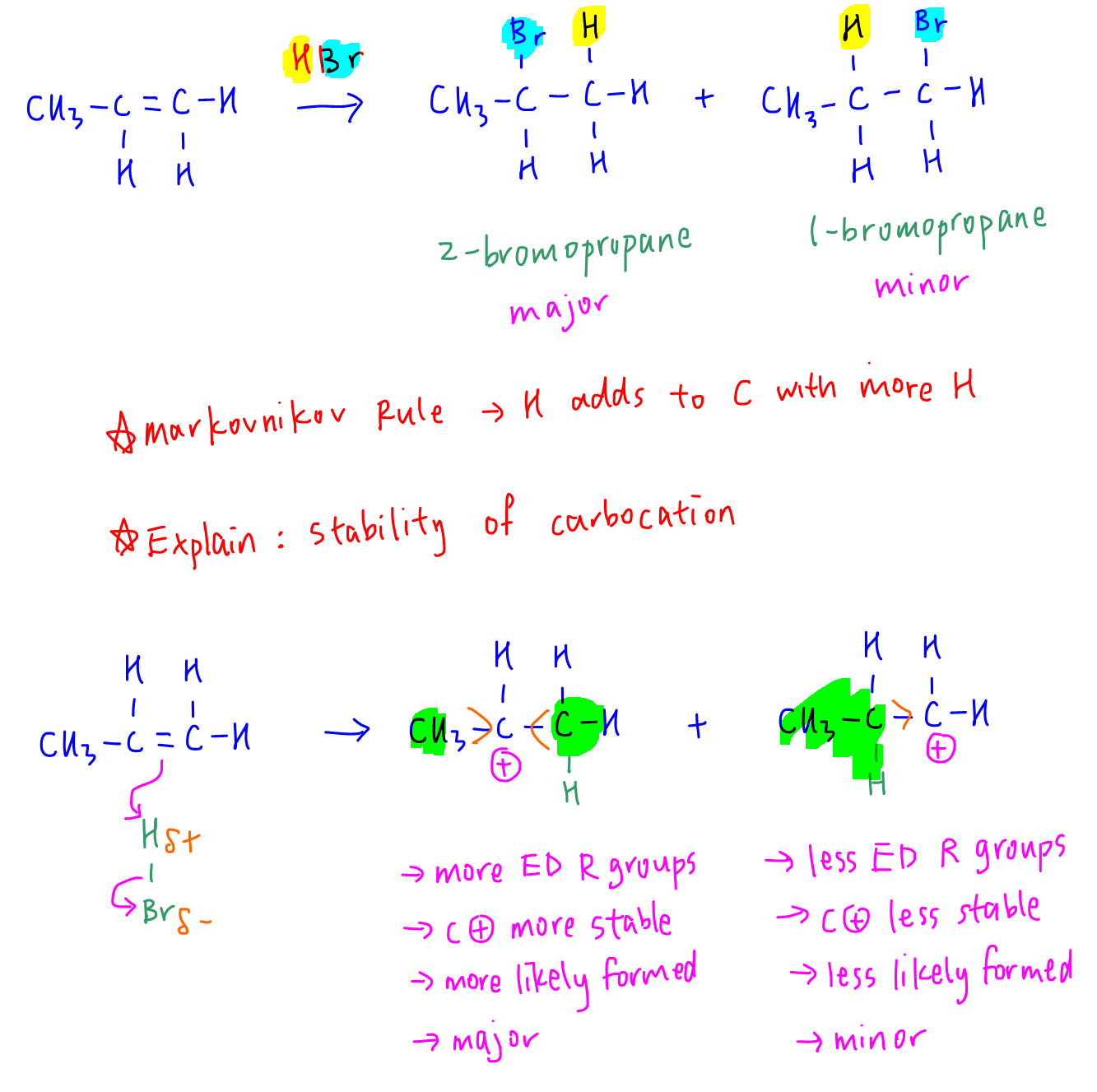
Hence Factor 1 is true since methyl group is electron donating, and factor 3 is false since methyl group is not electron withdrawing.
Factor 2 is false. Even though methyl group has a steric effect, its steric hinderance will make it harder for Br- to attack the carbocation in the second step of EA mechanism, hence disfavour the formation of 2-bromopropane.
Back to other 2024 A Level Paper 1 Questions
Found this Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new Chemistry videos every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
