2024 A Level H2 Chemistry Paper 1 Solutions - Questions 1 to 10
Question 1

Answer: D
Topic: Atomic Structure
Explanation:
Cations must be positively charged so O2- and F- are eliminated straight away.
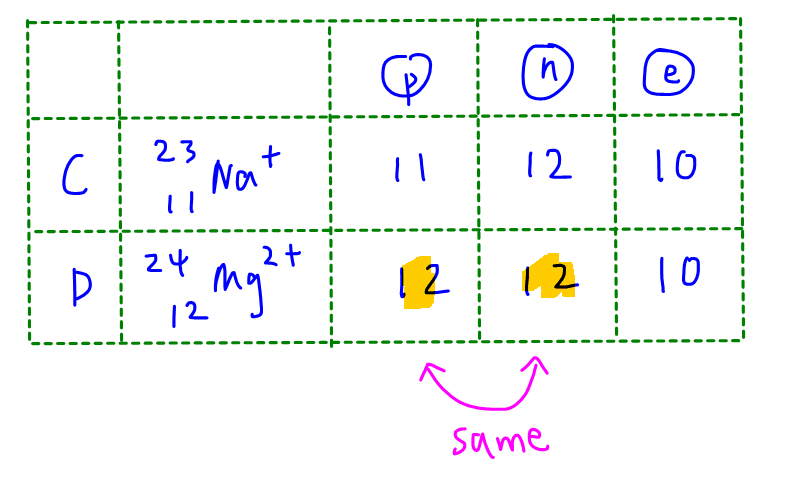
Both Na+ and Mg2+ have the same number of electrons as neon atom (10 electrons).
We calculate the neutron numbers for both Na+ and Mg2+ to determine that the proton number and neutron number for Mg2+ are the same.
Question 2

Answer: D
Topic: Atomic Structure
Explanation:
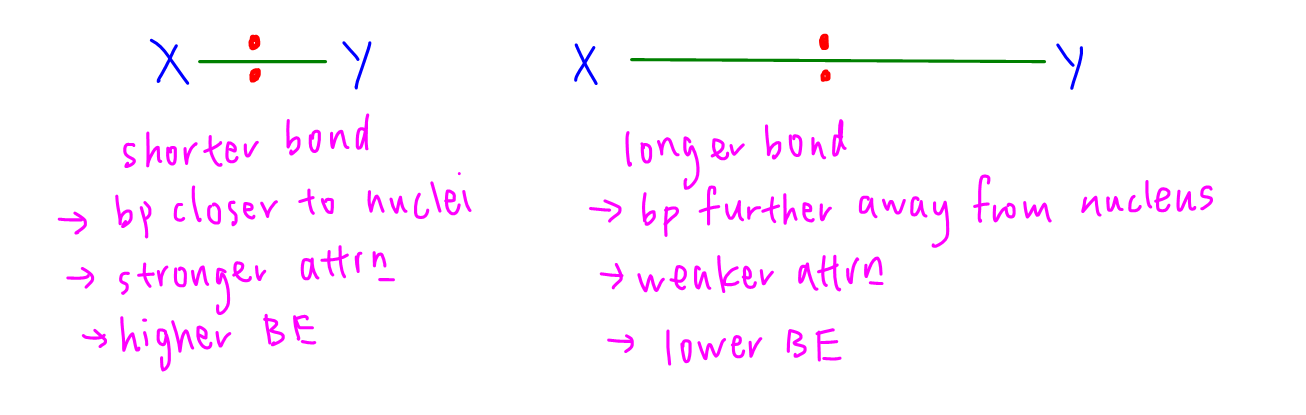
Statement 1 is false - in a longer covalent bond the bond pair of electrons are further away from the nuclei, attraction between nuclei and shared pair of electrons are weaker hence bond energy is lower.
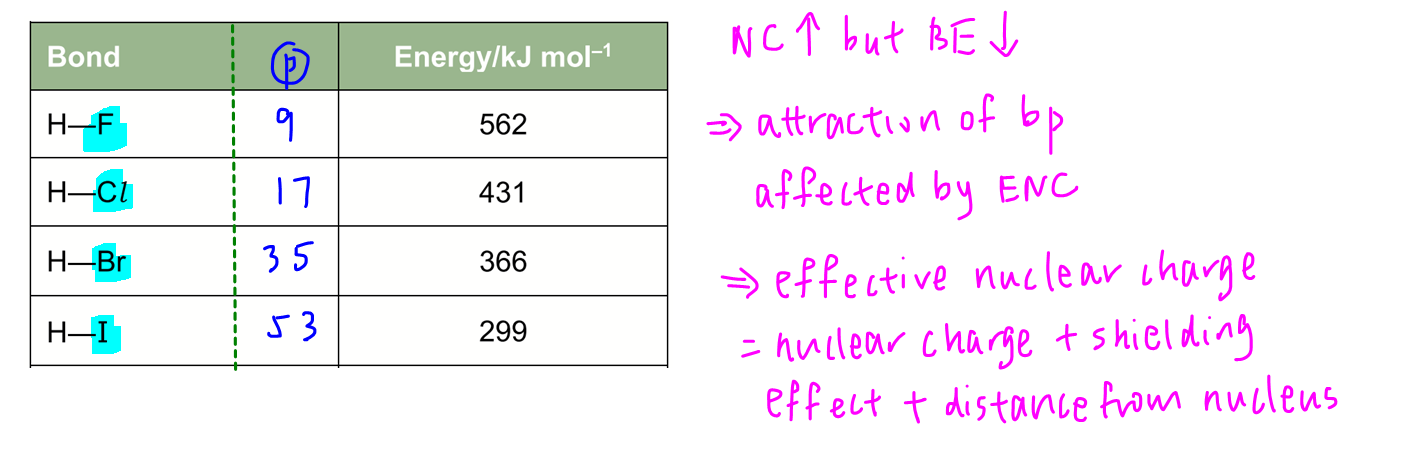
Statement 2 is false - effective nuclear charge not nuclear charge will affect overall attraction between nucleus and valence electrons and hence bond energy. For instance for hydrogen halides, down the group nuclear charge for halogen increases but bond energy for H-X decreases.
Statement 3 is true - attraction between shared electron pair and nuclei of bonded atoms is the definition of covalent bond hence stronger attraction means higher bond energy.
Question 3

Answer: C
Topic: Intermolecular Forces
Explanation:
Statement 1 is false - H2S is more polar so should have stronger pd-pd attraction than H2Se. Its lower boiling point is due to its smaller electron cloud size, polarisability and weaker id-id attraction.
Statement 2 is true - H2O is smaller in size, electron cloud less polarisable hence temporary dipole - induced dipole attraction is weaker than H2Se. Note that id-id attraction is present in any species with electron cloud, even if dominant IMF is pd-pd or H-bond (in the case for H2O).
Statement 3 is true - H2O is more polar so its pd-pd attraction is stronger than H2S. Note H-bond is a very strong type of pd-pd attraction.
Question 4
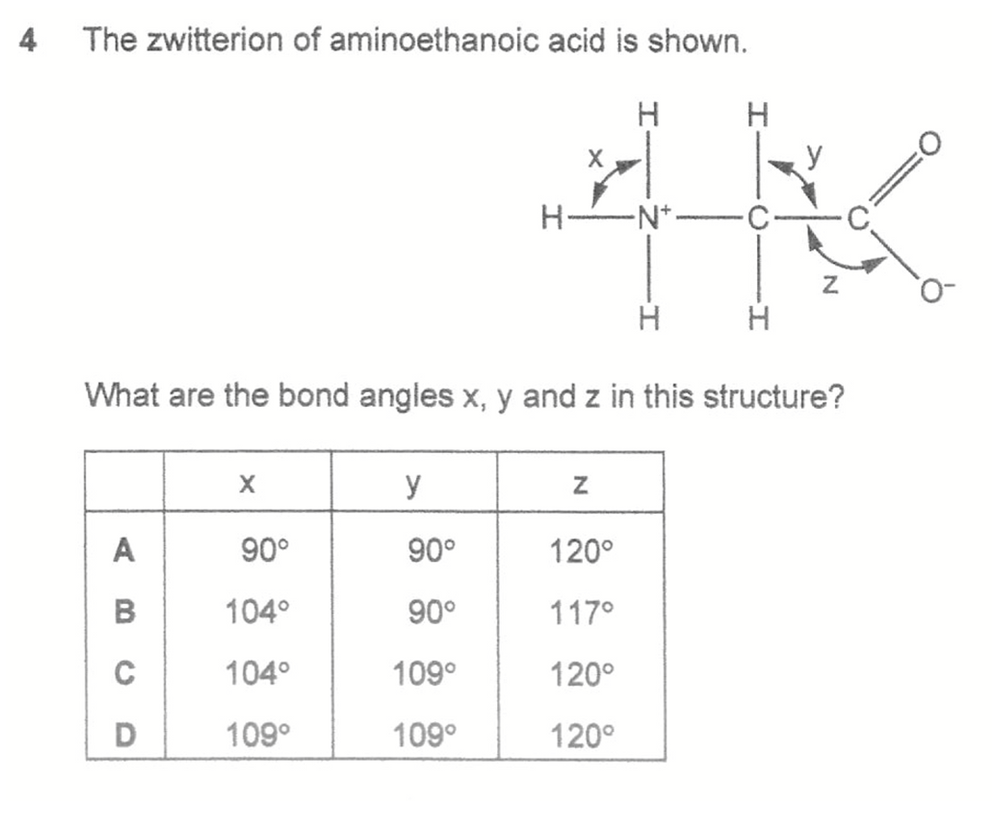
Answer: D
Topic: Chemical Bonding
Explanation:
We can use VSEPR theory to deduce the shape and bond angle based on the number of bond pairs and lone pairs attached to the central atoms.
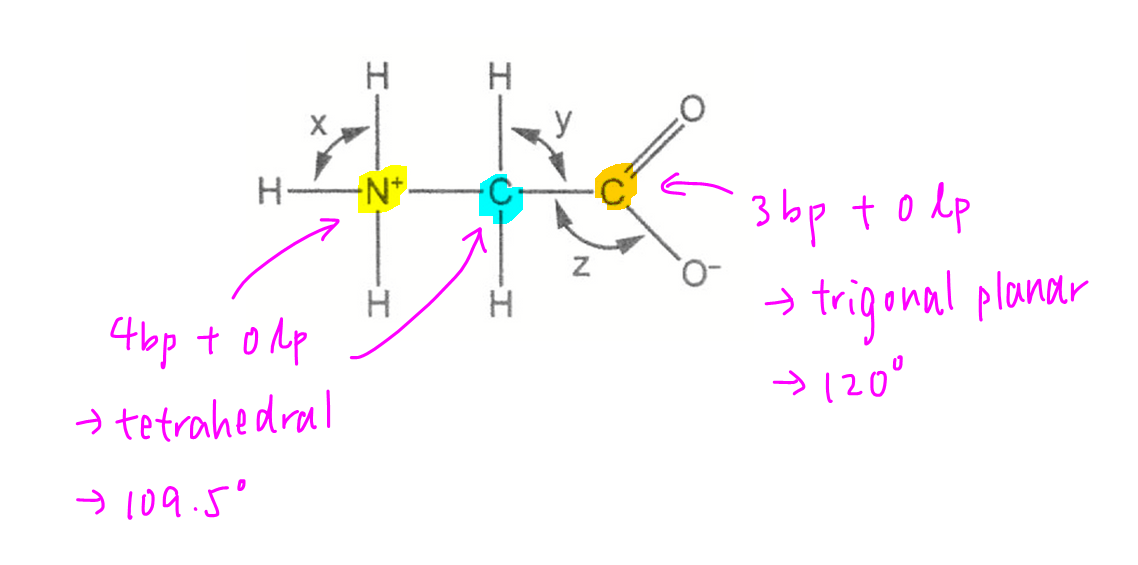
Ammonium N and alkyl C - 4 bp and 0 lp, shape tetrahedral, bond angle 109.5 deg.
Acid C - 3 bp and 0 lp, shape trigonal planar, bond angle 120 deg.
Question 5

Answer: B
Topic: Atomic Structure
Explanation:
We consider the electron in box diagram of valence shell (n=3) for Na to Ar and count the number of unpaired electrons.
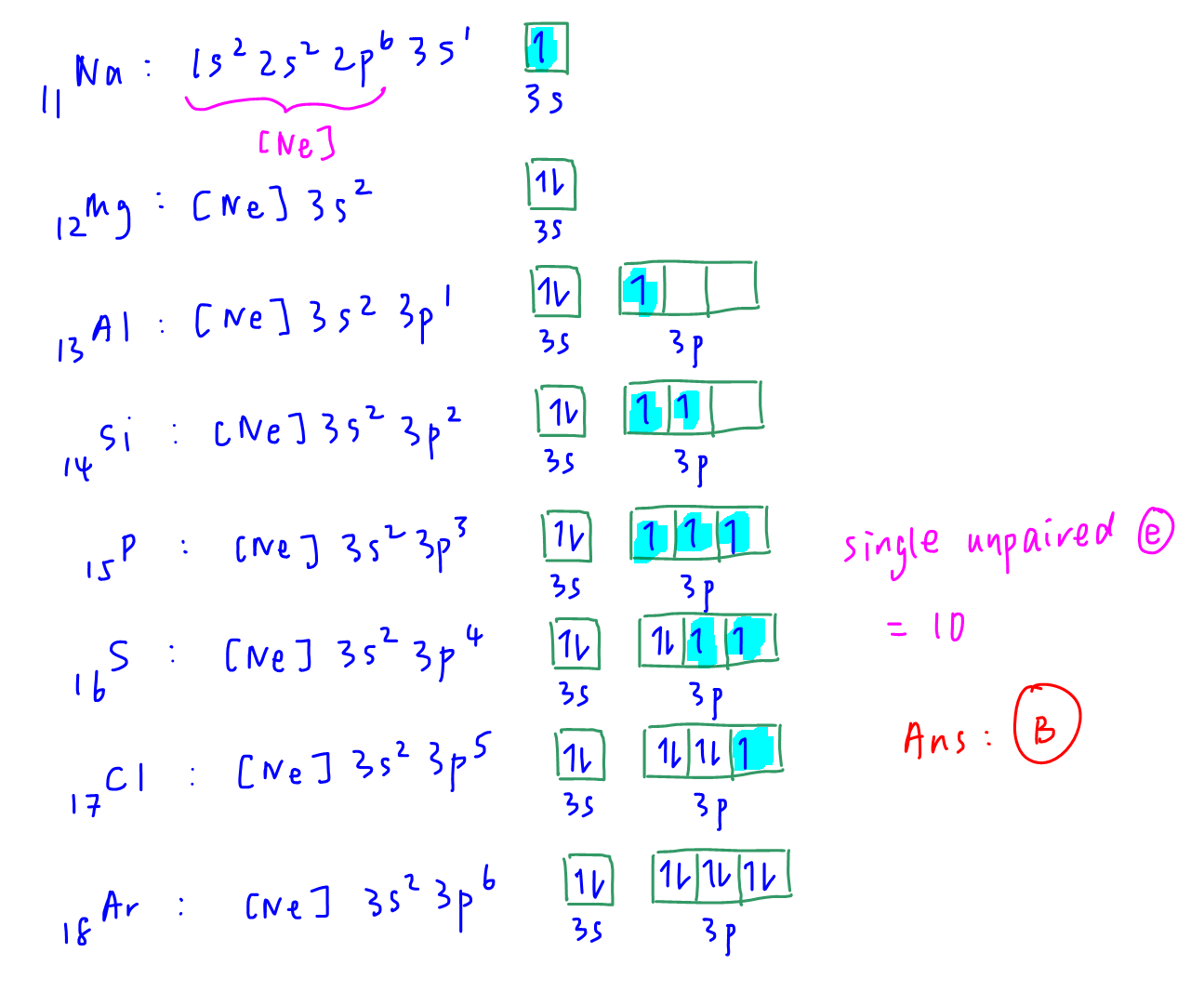
Question 6

Answer: B
Topic: Mole Concept
Explanation:
For each of the compounds, we use the mass analysis to determine its formula and oxidation state of Si, P or S. Their highest oxidation state should be +4, +5 and +6 respectively.
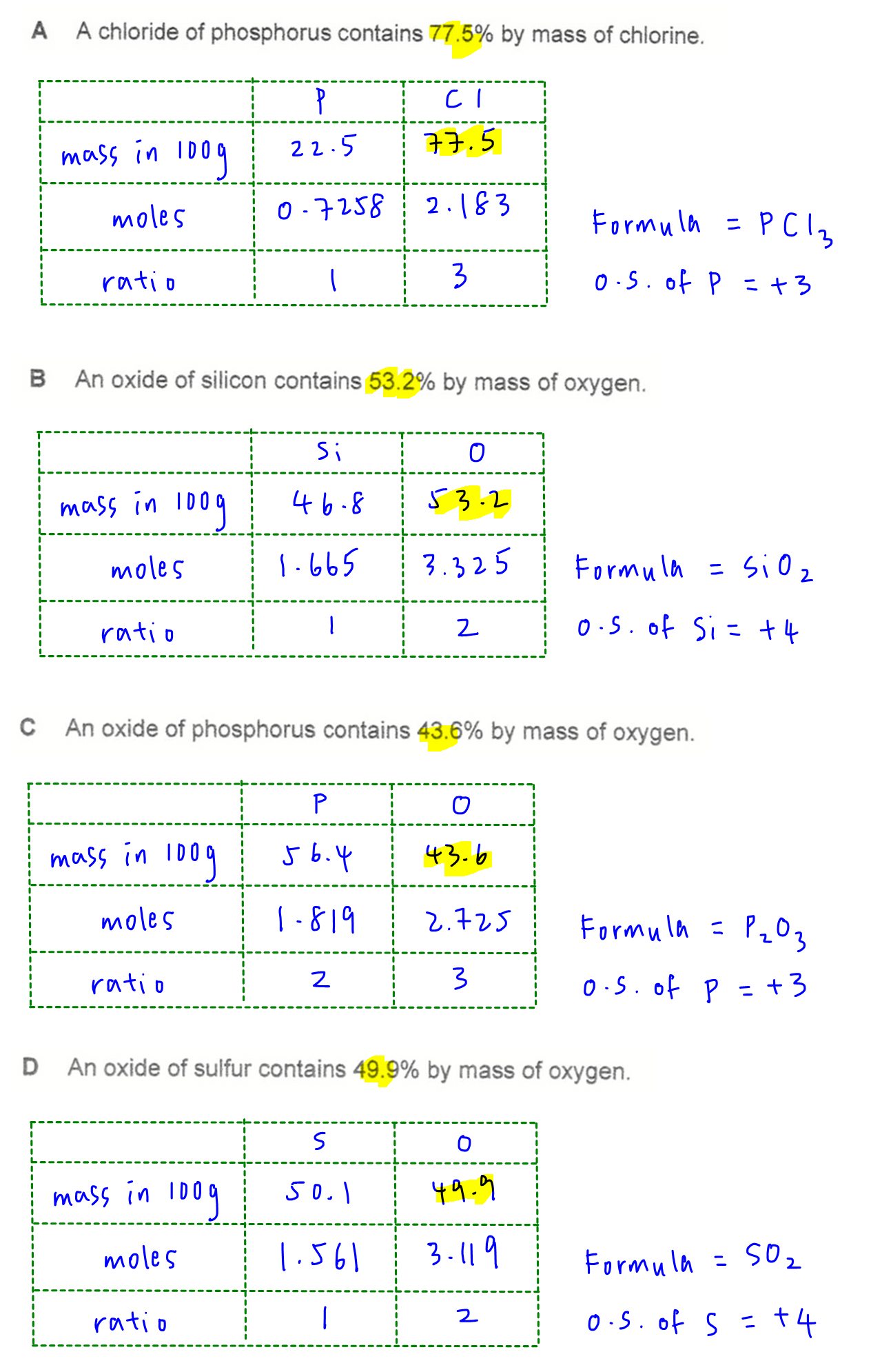
Question 7
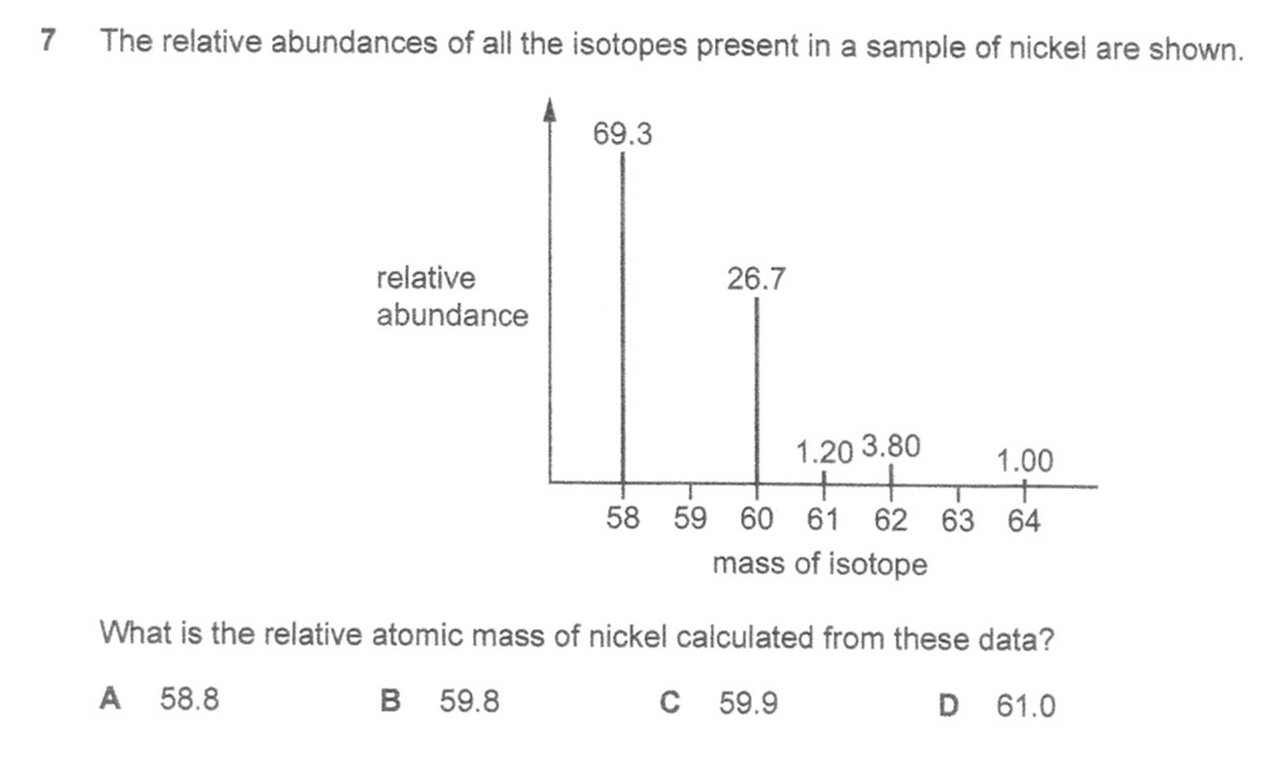
Answer: A
Topic: Mole Concept
Explanation:
Relative atomic mass is the sum of isotopic mass*abundance divided by total abundance.
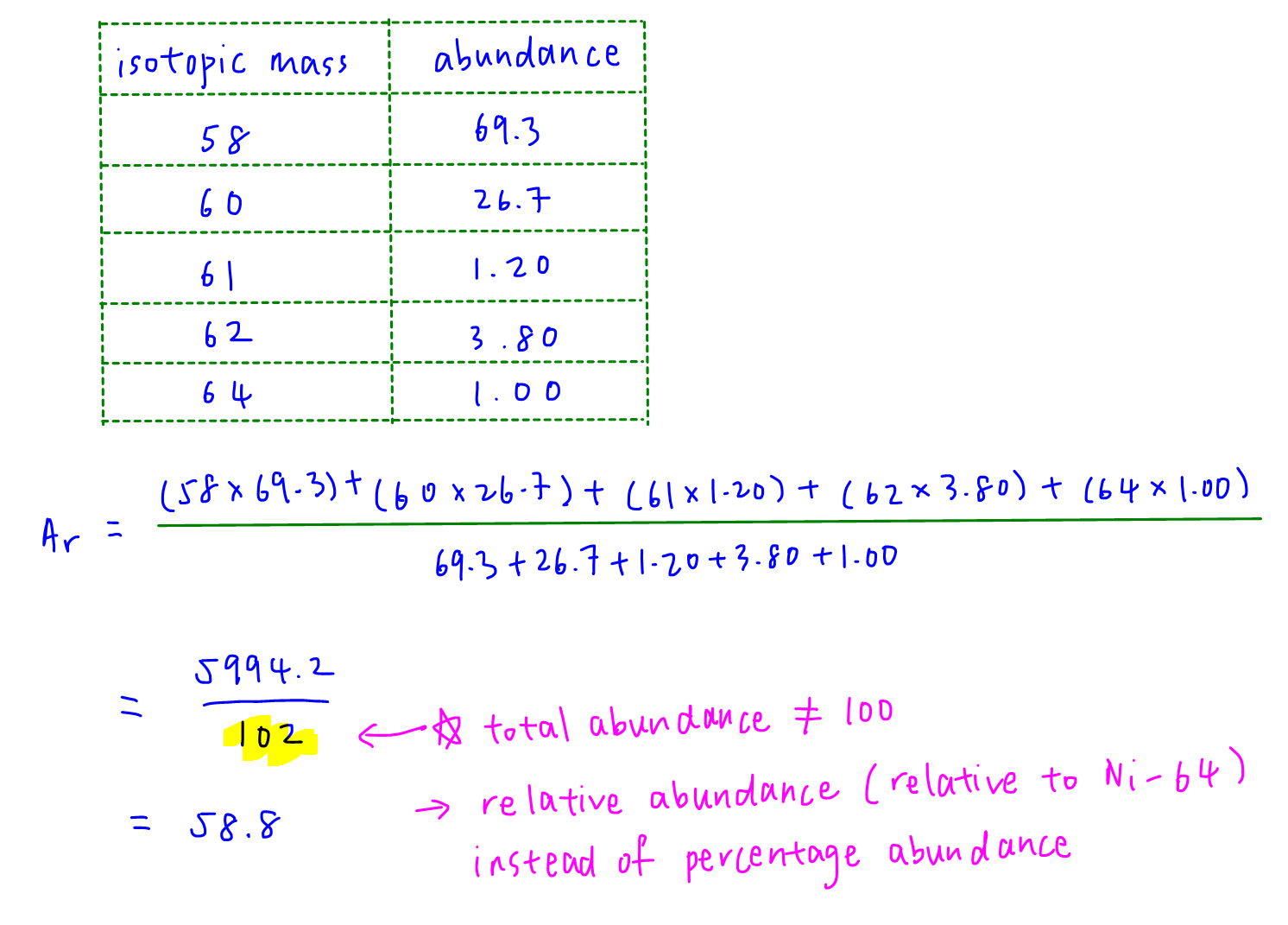
Note that the total relative abundance is 102 and not the usual total percentage abundance of 100.
Question 8
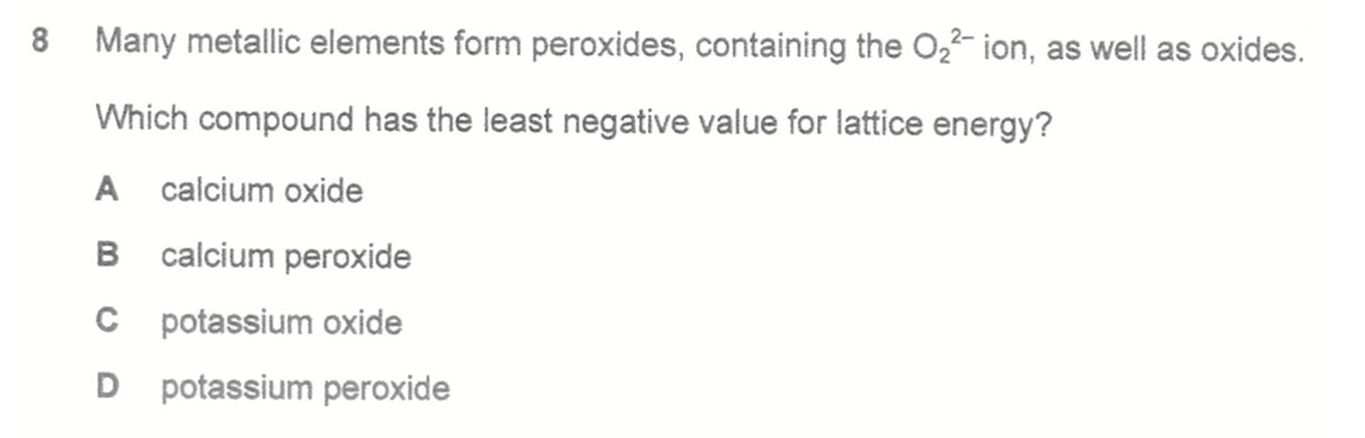
Answer: D
Topic: Chemical Bonding
Explanation:
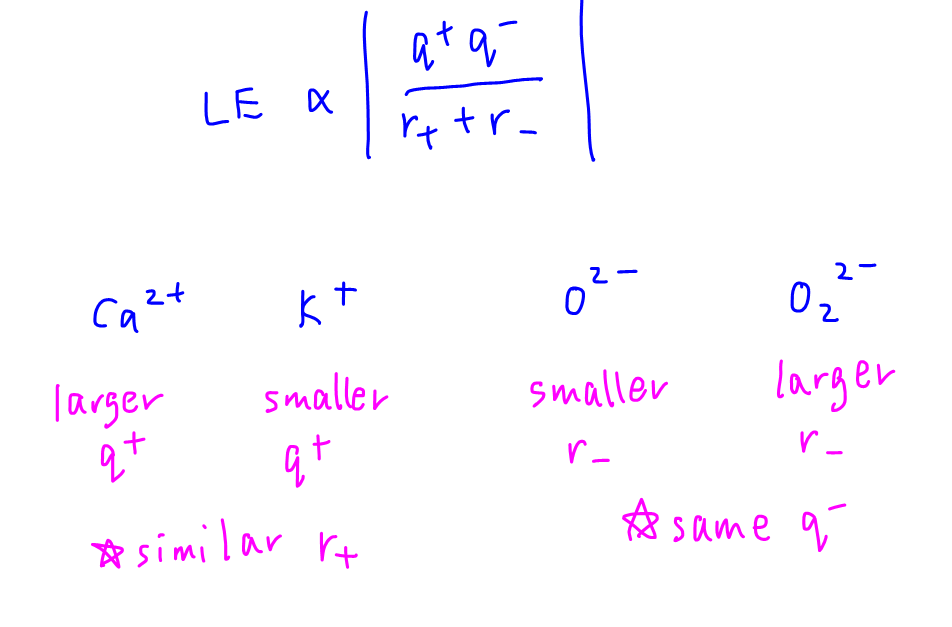
To get the smallest magnitude for lattice energy, we want charge of ion to be smaller and size to be larger. Hence potassium peroxide.
Question 9

Answer: C
Topic: Kinetics
Explanation:
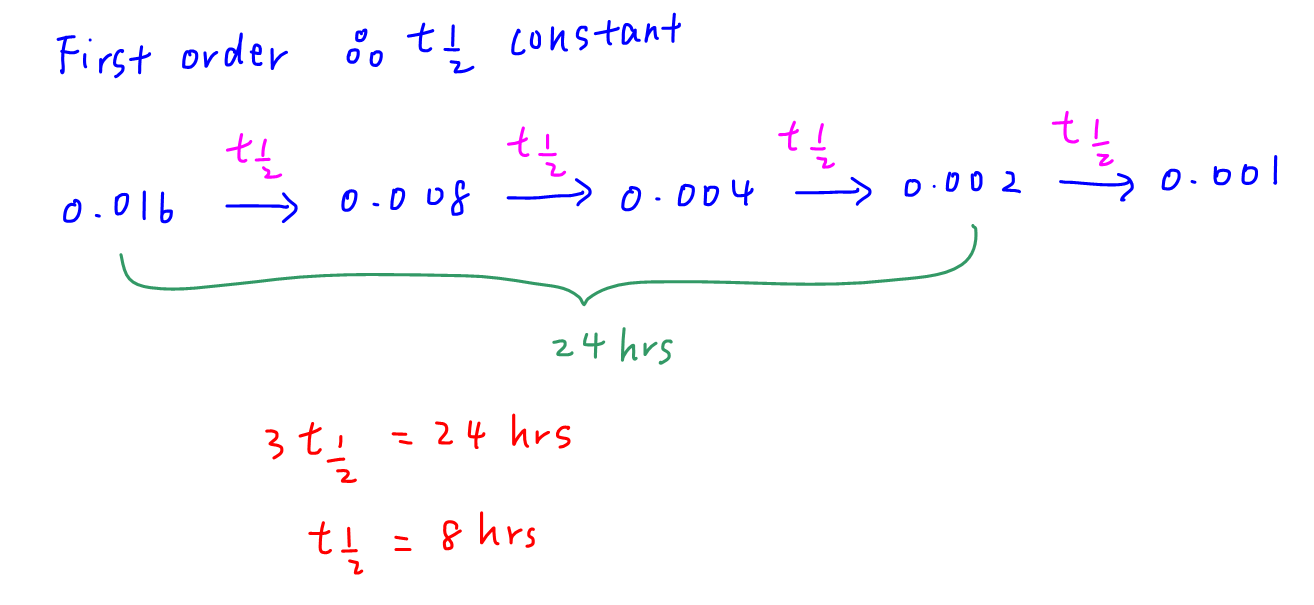
24 hours has passed from 0.016 mol dm-3 to 0.002 mol dm-3 which is 3 half lifes. Hence each half life is 8 hours and time taken to decrease from 0.016 mol dm-3 to 0.001 mol dm-3 is 4 half lifes which takes 32 hours.
Question 10
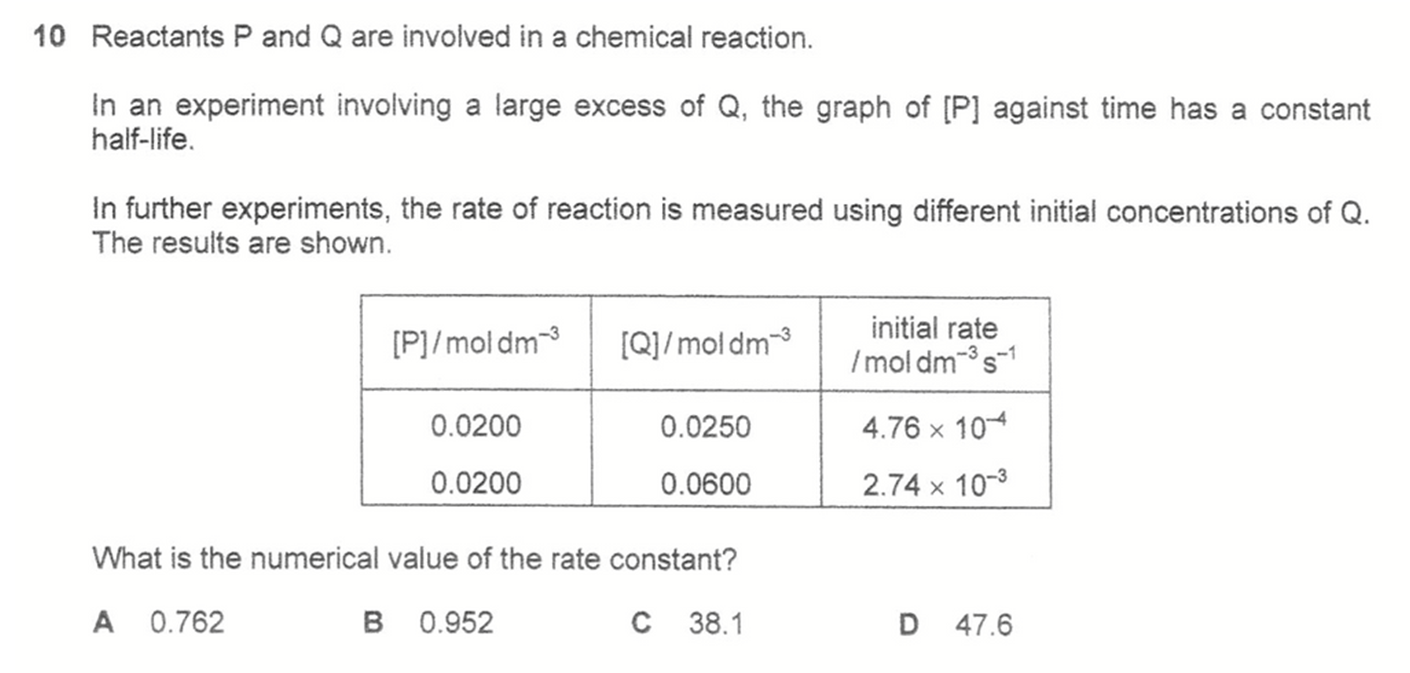
Answer: C
Topic: Kinetics
Explanation:
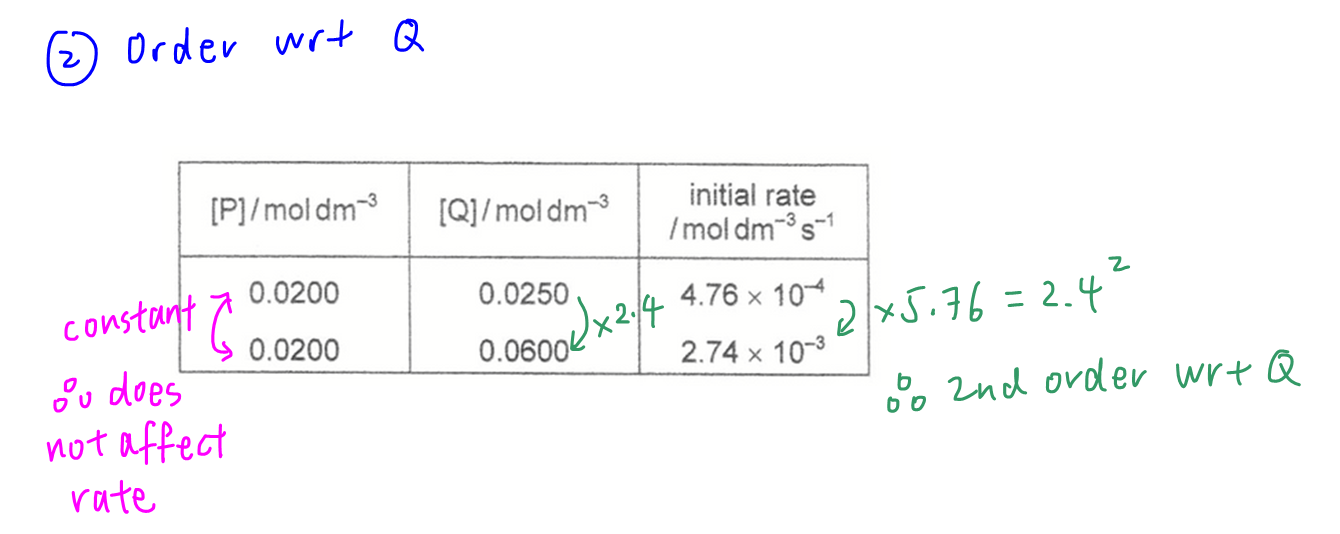
Graph of [P] against time has constant half-life hence first order with respect to P.

Comparing initial rates for the 2 experiments, [Q] increases by 2.4 times and initial rate increases by 5.76 times hence second order with respect to Q.
Hence rate = k [P][Q]2 and rate constant k can be calculated.
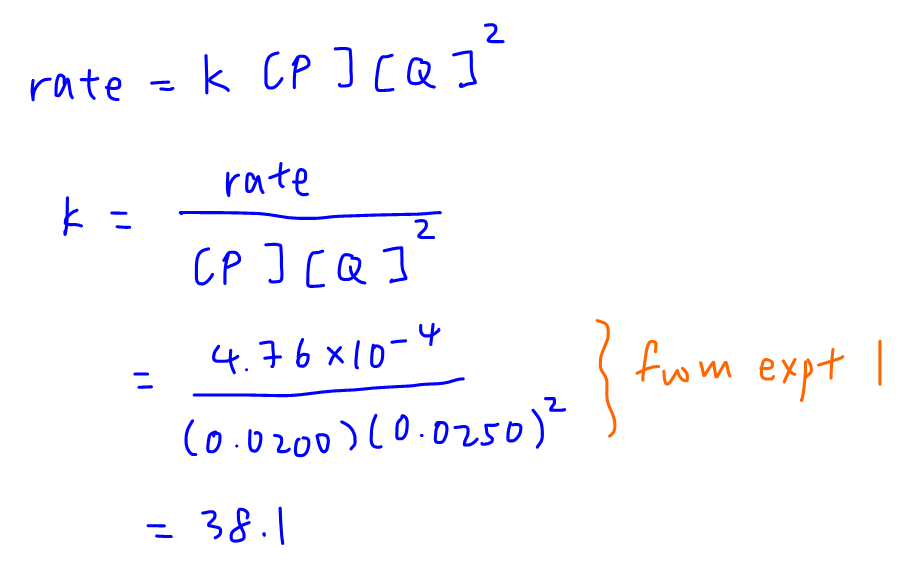
Back to other 2024 A Level Paper 1 Questions
Found this Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new Chemistry videos every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
