2024 A Level H2 Chemistry Paper 1 Solutions - Questions 21 to 30
Question 21
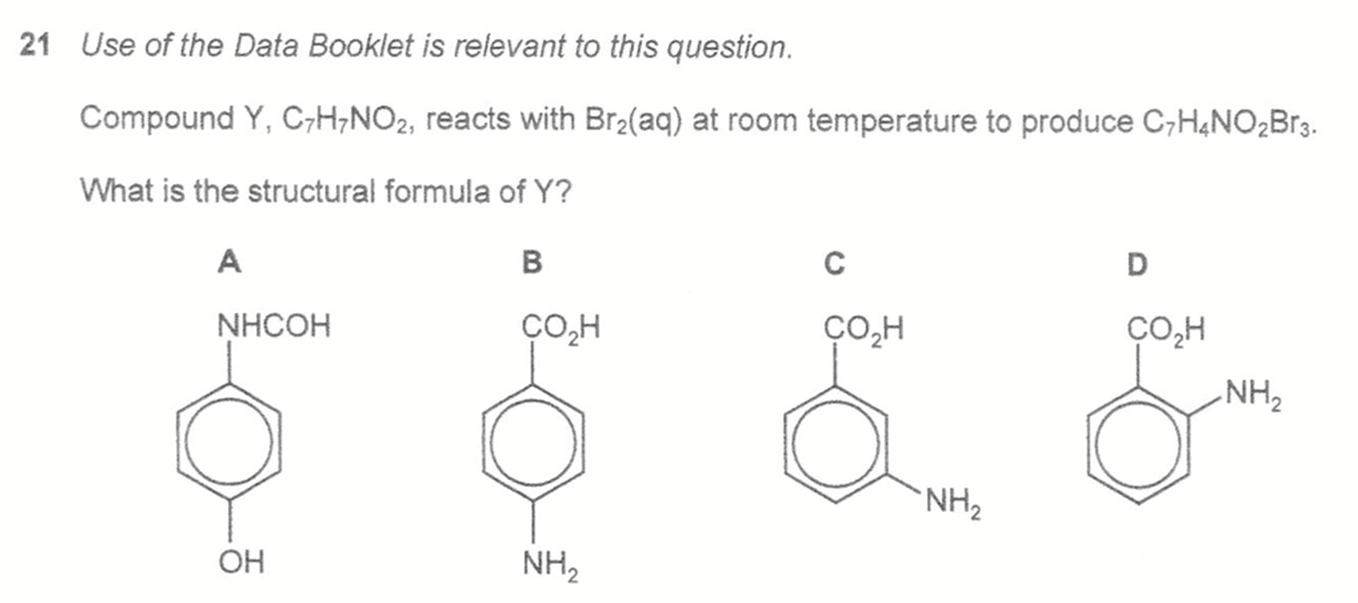
Answer: C
Topic: Phenol / Phenylamine
Explanation:
By comparing molecular formula for Y (C7H7NO2) and product (C7H4NO2Br3) we can deduce there are 3 H substituted by 3 Br due to the presence of highly activating -OH or -NH2 group to benzene.
Hence we can identify the highly activating group and determine which one will have tri-substitution.

Question 22
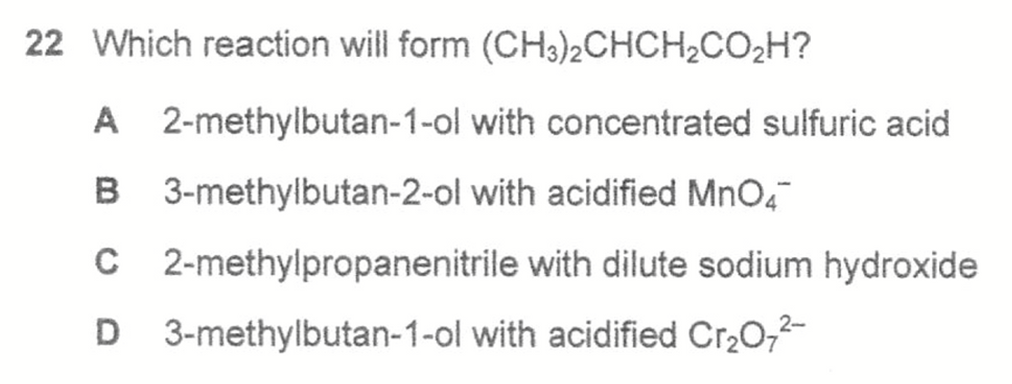
Answer: D
Topic: Organic Reactions
Explanation:
For each option we determine the product and deduce which is the required compound.
Type of Reactions:
A - elimination of water to form alkene
B - oxidation to form ketone
C - alkaline hydrolysis to form salt of acid
D - oxidation to form acid

Question 23

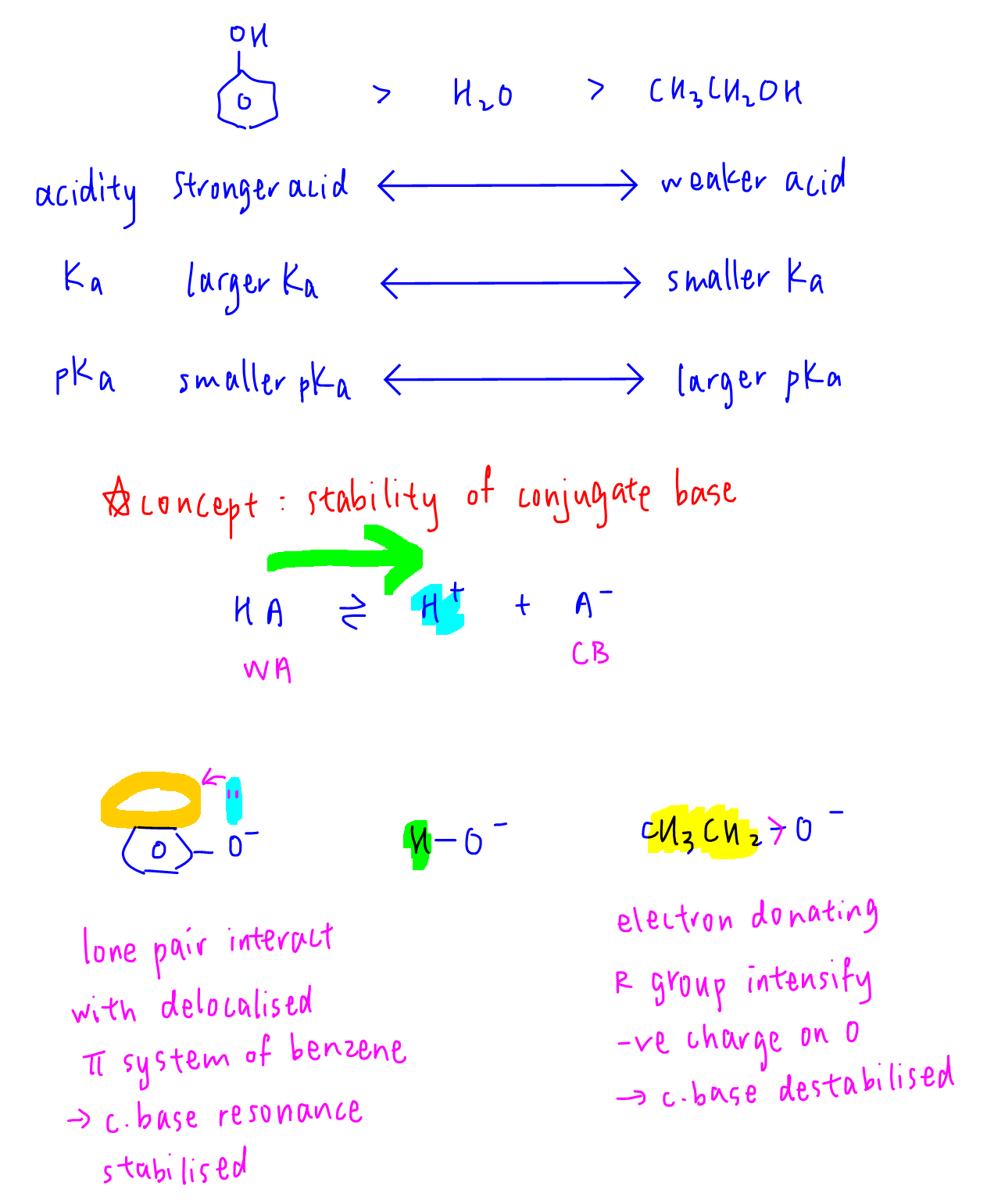
Answer: C
Topic: Acidity of Alcohols and Phenol
Explanation:
Comparing phenol, water and ethanol, phenol is the most acidic (largest Ka, smallest pKa) while ethanol is the least acidic (smallest Ka, largest pKa).
Acidity of organic compounds is explained via stability of conjugate base.
Phenoxide is stabilised by resonance when lone pair of oxygen interacts with delocalised pi system of benzene, hence phenol is more acidic than water.
Ethoxide is destabilised as electron donating ethyl group intensifies negative charge on oxygen, hence ethanol is less acidic than water.
Question 24

Answer: B
Topic: Organic Reactions
Explanation:
LiAlH4 is a source of hydride [H-] which is negatively charged. Therefore it will react with carbonyl carbon which is partial positive charge but not alkene which is electron rich.

Statement 1 is true since C=O bond is polar and carbonyl carbon is partial positive.
Statement 2 is false as alkenes should react with electrophiles in first step of addition.
Statement 3 is false as ketones should be reduced to alcohols instead.
Question 25

Answer: D
Topic: Amide Hydrolysis
Explanation:
Amides will undergo alkaline hydrolysis to form amine and salt of acid.

Question 26

Answer: A
Topic: Amino Acids
Explanation:
With excess hydroxide ions, the alpha acid will react to form carboxylate which is negatively charged. Hence the overall species should be negatively charged.

Question 27

Answer: C
Topic: Electrolysis
Explanation:
We can use the Faraday's equations to calculate charge and moles of electrons.
Then we can find moles and mass of copper reduced at the cathode.

Question 28

Answer: B
Topic: Electrochemistry
Explanation:
We can pull the half equations and E values for Br2/Br- and O2/H2O from the data booklet and calculate Ecell to be positive. This means reaction between O2 and Br- in acidic medium should be feasible under standard conditions.

Statement 1 is correct. In air the partial pressure of oxygen is smaller than 1 atm. Position of equilibrium for O2/H2O half equation shifts left and Er becomes less positive. This will give a less positive Ecell and make the reaction less feasible.

Statement 2 is likely correct. If activation energy is high, the rate of the reaction will be very slow and kinetically not feasible.
Statement 3 is incorrect. A higher concentration of Br- will shift position of equilibrium for Br2/Br- half equation to the left, Eo becomes less positive and Ecell becomes more positive. This means the reaction should become more feasible instead.

Question 29

Answer: C
Topic: Transition Elements
Explanation:
The d-d splitting pattern for octahedral complexes is 2 on top and 3 at the bottom.
For each cation we determine the number of d-electrons, fill up the electron in box diagram and determine the number of electrons in the higher energy 3d orbitals.

Question 30

Answer: B
Topic: Electrochemistry
Explanation:
SO2 is oxidised to sulfate hence can reduce VO2+ to a certain extent.
For each possible redox reaction between SO2 and vanadium species, we calculate its Ecell. If positive, reaction is feasible and vanadium species will be reduced.
We can then consider the redox reaction between SO2 and this new vanadium species and determine its feasibility.
Final oxidation state of vanadium is determined when the Ecell of the reaction is negative, reaction is not feasible and hence no more change in oxidation state for vanadium.

Back to other 2024 A Level Paper 1 Questions
Found this Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new Chemistry videos every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
