Three Definitions of Acids and Bases
In this JC1 webinar we want to discuss the three definitions of acids and bases in A Level Chemistry.
The balanced equation for reaction between sulfuric acid and ammonia is as follows:
H2SO4 + 2NH3 → (NH4)2SO4
This is clearly an acid base reaction, but where is the water of neutralisation?
In order to understand this, we need to go through the different definitions of acids and bases.
1. Arrhenius Theory
This is the definition used in secondary schools and O Levels where:
Arrhenius acid - dissolves in water to give H+(aq)
Arrhenius base - dissolves in water to give OH-(aq)
Therefore any acid base reaction will simply be the reaction between H+ and OH- to give water.
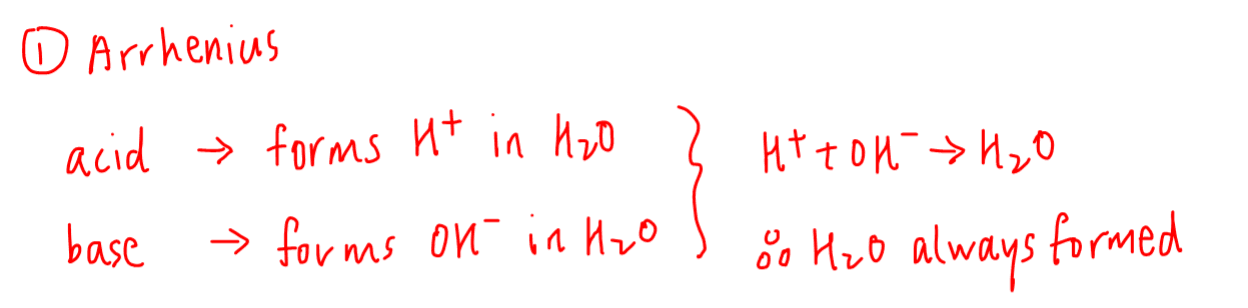
This definition is quite restrictive as the acid and base must be soluble in water.
Hence this will not apply to acid base reactions in gaseous systems or organic compounds which are not soluble in water.
2. Bronsted-Lowry Theory
This is the default definition used in A Levels where:
Bronsted acid - H+ or proton donor
Bronsted base - H+ or proton acceptor
Bronsted acid and base need not be soluble in water, hence will be more general than Arrhenius theory.
The major difference is Bronsted base just need to be a H+ acceptor and forming OH- is not required.
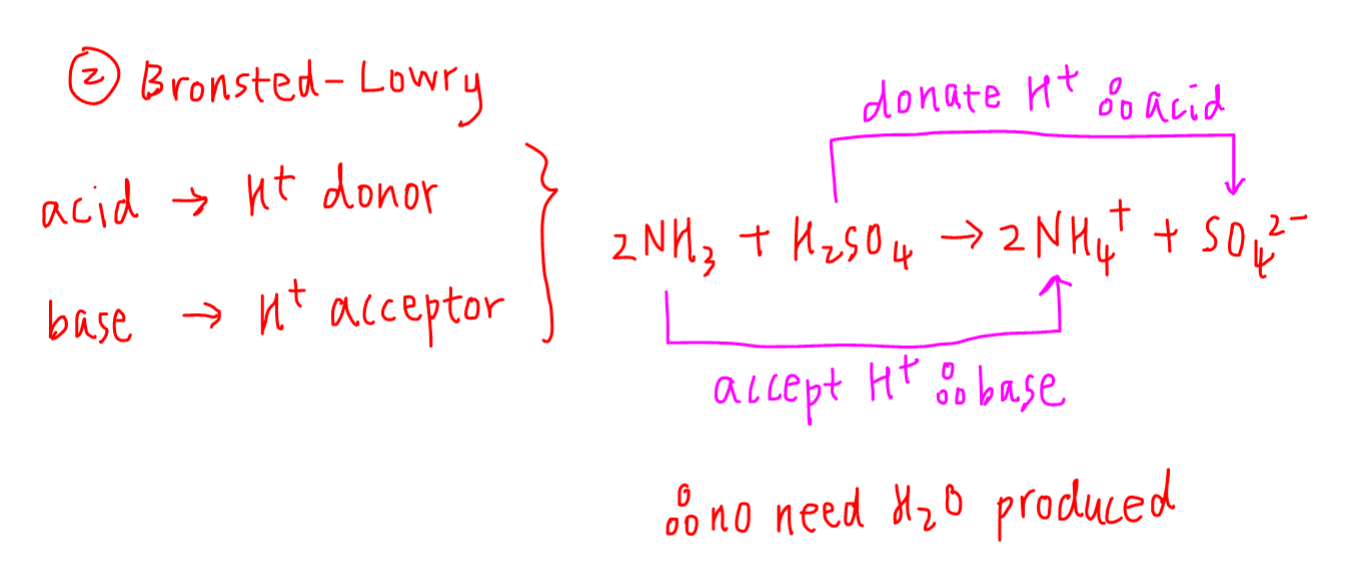
For the example given earlier, we can see that H2SO4 is the proton donor hence Bronsted acid, while NH3 is the proton acceptor hence Bronsted base.
Bronsted acid base reaction is just a transfer of H+ from acid to base so water need not be formed.
3. Lewis Theory
We use this definition sparingly in A Levels as it is the most abstract.
Lewis acid - electron pair acceptor
Lewis base - electron pair donor
Notice there is no need for the Lewis acid to form H+, hence it is harder to link this to Arrhenius theory.
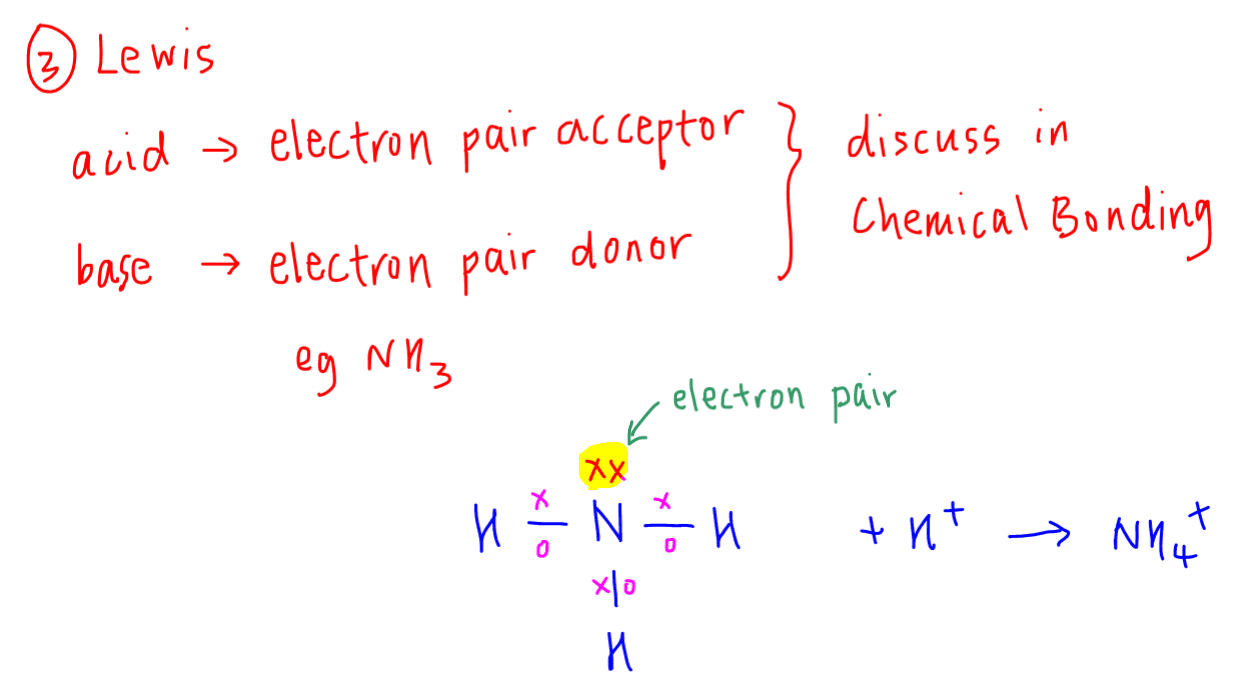
One way to remember is to use NH3 as an example of Lewis base.
Since nitrogen in NH3 has a lone pair of electrons to donate, a Lewis base is an electron pair donor.
Hence Lewis acid will be an electron pair acceptor.
Topic: Ionic Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
