3 Rules to write out Electronic Configuration using Quantum Model
In this JC1 webinar we want to learn how to write out electronic configuration using Quantum Model.
There are 3 rules that we can use.
1. Aufbau Principle
Electrons are first placed in the orbital of the lowest energy, then the orbital of the next lowest energy and so on.
This idea is exactly the same as Bohr's Model, where we fill electrons to the first shell first which has the lowest energy, then the second shell, and so on.
The distance from the nucleus and energy level of subshells from principle quantum shells n=1 to n=4 are shown below:
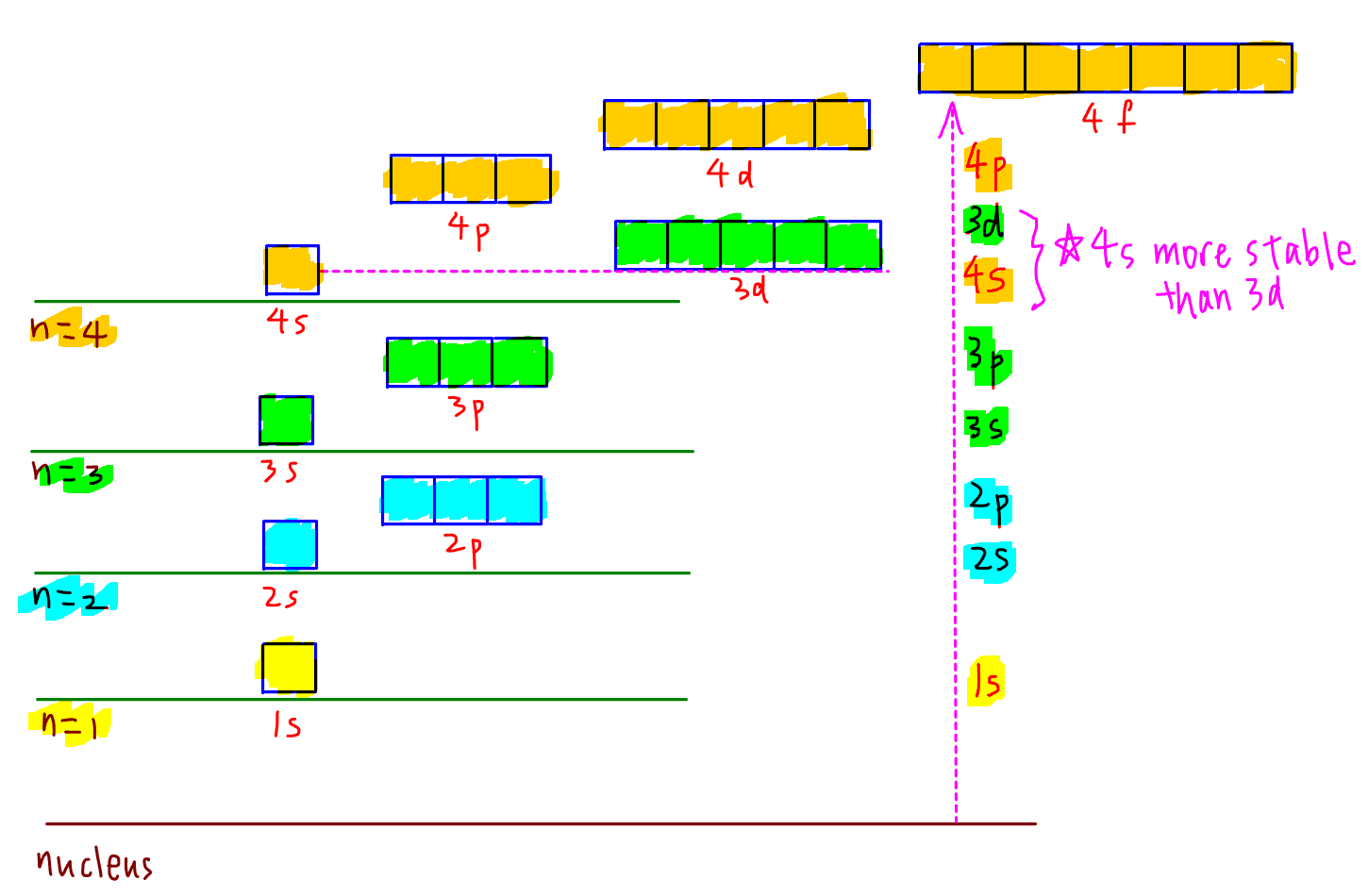
The subshells in increasing energy and order of filling electrons will therefore be:
1s 2s 2p 3s 3p 4s 3d 4p ...
Notice 4s subshell is more stable or closer to the nucleus than 3d subshell when empty.
Hence we add electrons to 4s subshell first, then to 3d subshell.
We can also determine the maximum number of electrons each subshell can hold.
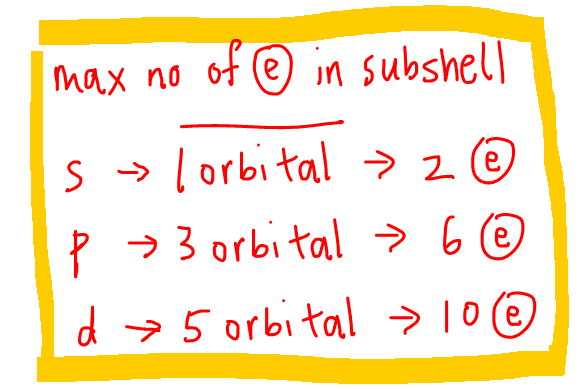
- s subshell with 1 orbital can hold 2 electrons
- p subshell with 3 orbitals can hold 6 electrons
- d subshell with 5 orbitals can hold 10 electrons
2. Pauli Exclusion Principle
Each orbital can only hold a maximum of 2 electrons.
If there are 2 electrons in the same orbital, they must have opposite spins.
So these are the allowed and not allowed configurations we can have for an orbital.

3. Hund's Rule
Electrons are added to orbitals in a subshell singly and with parallel spins before orbitals are occupied in pairs.
Using p subshell as an example, the first electron can be placed in the first orbital with spin up.
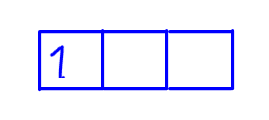
Second electron should be placed in another empty orbital with the same spin as the first one to minimise repulsion.
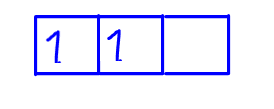
Third electron should be placed in the last empty orbital with same spin.
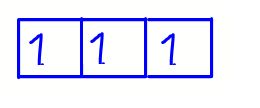
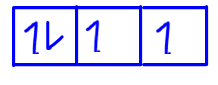
Fourth electron now has to be paired with another electron in the first orbital so it has to be spin down or of opposite spin.
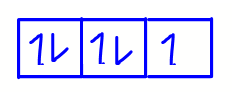
Fifth electron will go to the second orbital with spin down.
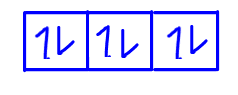
Finally the sixth electron will go to the third orbital with spin down.
A combination of these 3 rules will allow us to write out electronic configurations of atoms or ions using the Quantum Model.
Topic: Atomic Structure, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
