A Level Chemistry 2017 Paper 1 Question 1 Solution
In this video we want to discuss 2017 A Level H2 Chemistry Paper 1 Question 1.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through the question.
Topic tested is on Mole Concept, in particular how to determine Relative Atomic Mass of an element from abundances of different isotopes.
Each element will have different isotopes with different relative abundances.
For example, chlorine has isotopes Cl-35 and Cl-37.
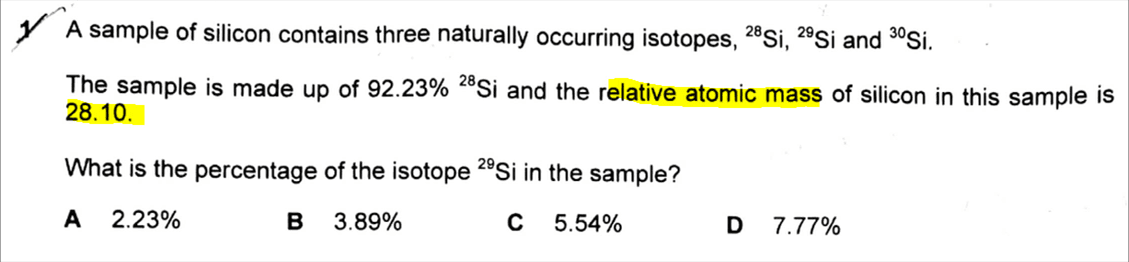
In the case of silicon, since the relative abundance of Si-28 is given to be 92.23%, we can then tabulate and figure out the relative abundances of the rest of the isotopes.
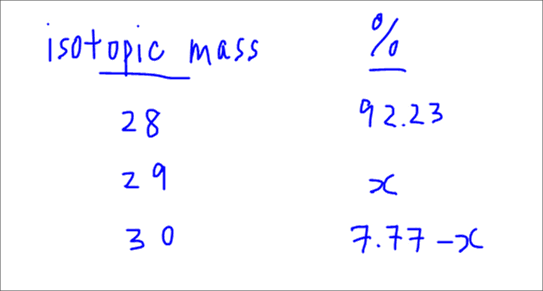
The relative atomic mass is the weighted average mass of all the isotopes, taking into account the relative abundances of the isotopes.
The method to calculate relative atomic mass is very simple:
1. Multiply the isotopic mass of each isotope by its relative abundance (in percentages)
2. Add all these products for all the isotopes
3. Divide this sum by 100%
We then have an equation involving unknown abundance x and hence can solve for x.
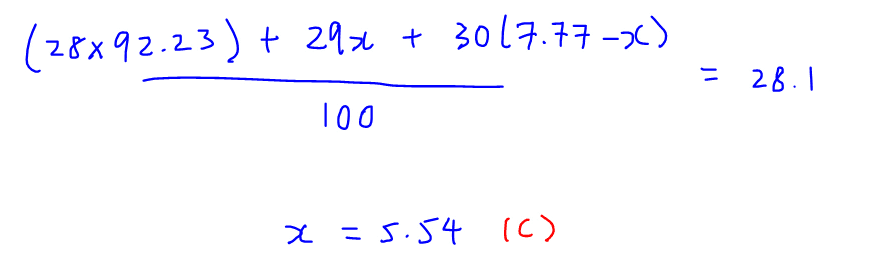
We can then conclude that percentage abundance of Si-29 is 5.54% hence the answer to this question is C.
Check out this video for the question and the suggested solution!
Topic: Mole Concept, Physical Chemistry, A Level Chemistry, Singapore
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Check out other A Level Chemistry Video Lessons here!
Looking for H2 Chemistry Tuition? Do consider taking up my classes at Bishan or online classes!
