A Level Chemistry 2017 Paper 1 Question 14 Solution - Exclusive
In this exclusive video we want to discuss the suggested solution for A Levels Chemistry (H2 Chemistry) 2017 Paper 1 Question 14.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this question:
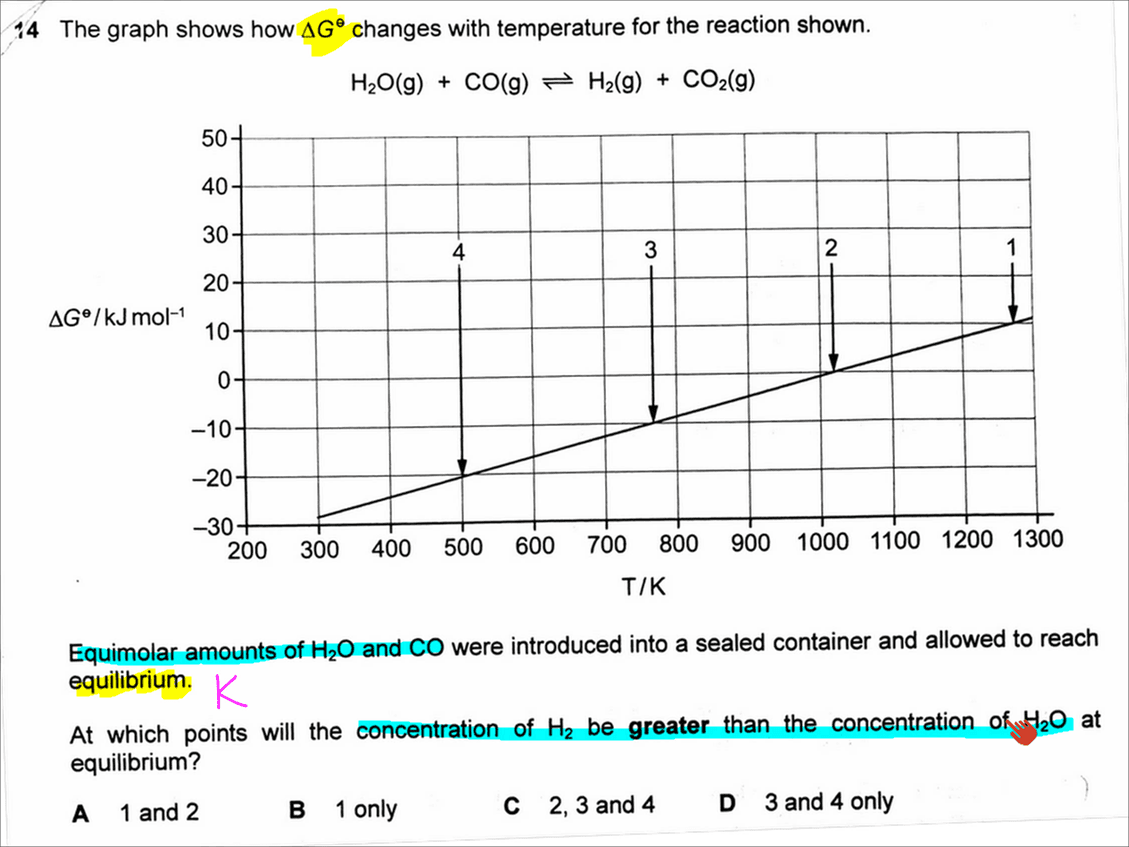
The topic tested in this question is on Chemical Equilibria. In particular the relationship between standard Gibbs free energy and equilibrium constant K given by the expression:

First we need to write out the K expression from the balanced equation.

Equimolar amounts of reactants H2O and CO are used and the mole ratio of reactants and products are all the same.
This means the concentration of reactants H2O and CO will remain the same, and the concentration of products H2 and CO2 will remain the same as well.
We need to determine when would concentration of product H2 be greater than concentration of reactant H2O. This means that K must be greater than 1.
We can substitute K > 1 back into the Gibbs free energy expression and deduce that standard Gibbs free energy must be negative.

We can then look back at the graph to determine that points 3 and 4 are at negative standard Gibbs free energy. So the answer to this question has to be D.

Check out this video for the full solution and detailed calculation!
Topic: Chemical Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
You might also be interested in the suggested solution for Paper 1 Question 13.
Check out other A Level Chemistry Video Lessons here!
Looking for H2 Chemistry Tuition? Do consider taking up my classes at Bishan or online classes!
