A Level Chemistry 2017 Paper 1 Question 15 Solution - Exclusive
In this exclusive video we want to discuss the suggested solution for A Levels Chemistry (H2 Chemistry) 2017 Paper 1 Question 15.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this question:
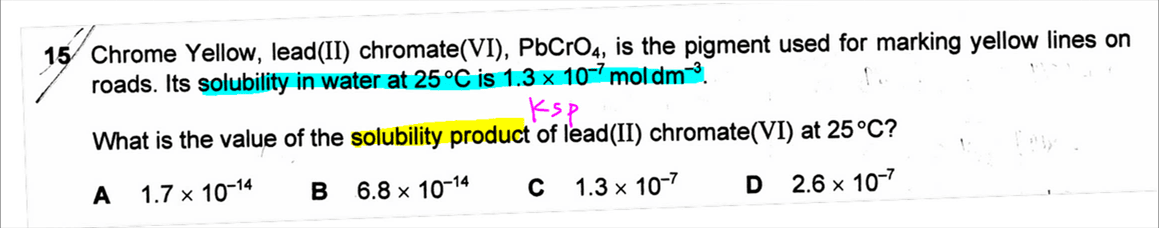
The topic tested in this question is on Solubility Product.
We can first write down the dissociation of sparingly soluble salt PbCrO4 and let x be the solubility of this salt.
So at equilibrium the concentration of Pb2+ and CrO42- will both be x.
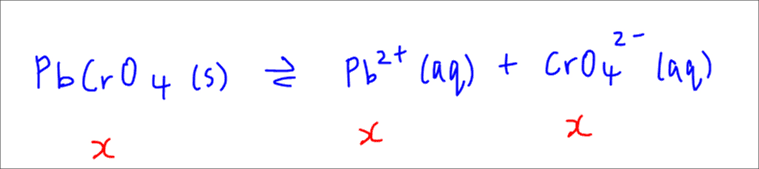
We can then express the Ksp in terms of solubility and solve for Ksp since solubility x is known.

We can then compare the answer to the options and determine the answer is A.
Check out this video for the full solution and detailed calculation!
Topic: Solubility Product, Physical Chemistry, A Level Chemistry, Singapore
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
You might also be interested in the suggested solution for Paper 1 Question 14.
Check out other A Level Chemistry Video Lessons here!
Looking for H2 Chemistry Tuition? Do consider taking up my classes at Bishan or online classes!
