A Level Chemistry 2017 Paper 1 Question 17 Solution - Exclusive
In this exclusive video we want to discuss the suggested solution for A Levels Chemistry (H2 Chemistry) 2017 Paper 1 Question 17.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this question:

The topic tested in this question is on Electrochemistry. In particular the concept of electrolysis and calculation of products discharged during electrolysis.
We first need to deduce the products formed at the anode and cathode.
The reaction at the cathode is already given in the question where acrylonitrile is reduced.
For the reaction at the anode, water will be oxidised to form O2.
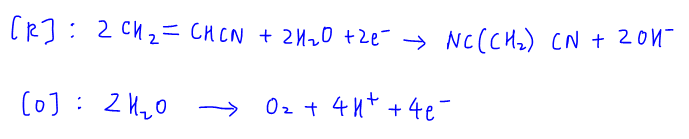
Since 0.01 mol of acrylonitrile is electrolysed, we can use the reduction half equation to compare mole ratio between electron and acrylonitrile to determine the number of moles of electrons gained at the cathode is 0.01 mol.
Since the electrolysis is the same redox reaction,
mol of electrons lost at the anode = mol of electrons gained at cathode = 0.01 mol

We can then compare mole ratio between O2 and electron to determine the number of moles of O2 produced is 2.5 x 10-3 mol.
Finally we can work out volume of O2 at room temperature and pressure by multiplying mol of O2 with molar volume at r.t.p. (24.0 dm3)
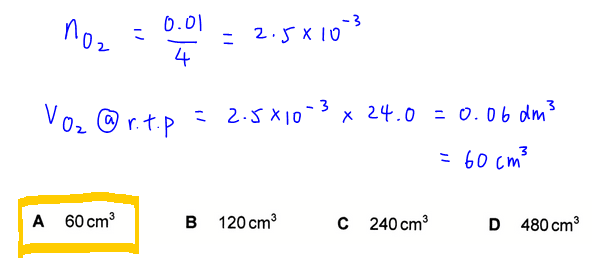
Therefore the answer to this question is A.
Check out this video for the full solution and detailed explanation!
Topic: Electrochemistry, Physical Chemistry, A Level Chemistry, Singapore
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
You might also be interested in the suggested solution for Paper 1 Question 16.
Check out other A Level Chemistry Video Lessons here!
Looking for H2 Chemistry Tuition? Do consider taking up my classes at Bishan or online classes!
