A Level Chemistry 2017 Paper 1 Question 19 Solution - Exclusive
In this exclusive video we want to discuss the suggested solution for A Levels Chemistry (H2 Chemistry) 2017 Paper 1 Question 19.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this question:
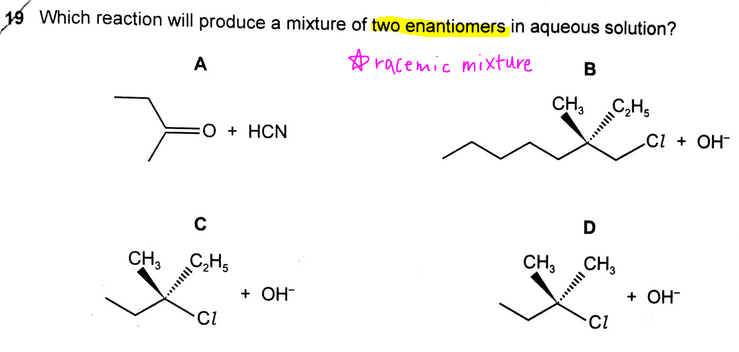
The topic tested in this question is on Nucleophilic Substitution Mechanism of Halogenoalkanes and Nucleophilic Addition Mechanism of Carbonyl Compounds.
In A Level Syllabus when a mixture of two enantiomers are formed they are usually in equimolar amounts. This means the product mixture will be optically inactive as the racemic mixture will cancel out the optical activity of each other.
We need to know the following mechanisms and the stereochemistry of the products if the starting compound contains a chiral carbon and is optically active.
Nucleophilic Substitution of Halogenoalkanes

If the halogenalkane is chiral and optically active, the stereochemistry of the product will depend on whether the reaction mechanism is via SN1 or SN2.
SN1 mechanism involves the formation of an sp2 hybridised carbocation. Since the carbocation is planar, the nucleophile can attack the carbon from both sides to equal extent. Product formed will be a racemic mixture and optically inactive.
SN2 mechanism involves exclusively the back-door attack of nucleophile from directly behind the C-X bond. There will only be 1 enantiomer product formed hence will be optically active.
Nucleophilic Addition of Carbonyl Compounds

Carbonyl carbon is sp2 hybridised. Therefore the scenario is similar to SN1 mechanism where the planar carbonyl carbon can be attacked from both sides to equal extent. If the carbonyl group is asymmetrical, the product formed will be a racemic mixture and optically inactive.
Considering the options
We can finally look through all the options and decide which will give a mixture of 2 enantiomers.
Option A involves the nucleophilic addition of an asymmetrical carbonyl group hence product formed will be racemic.
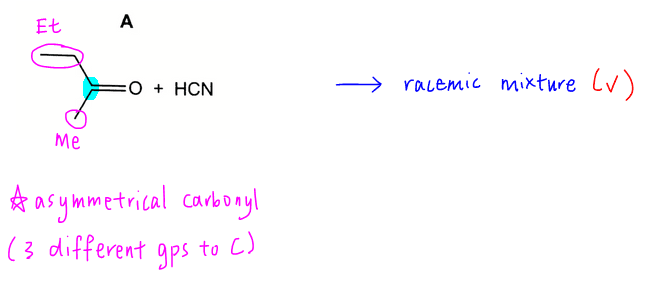
Options B to D involve the nucleophilic substitution of halogenoalkanes. All the carbons involved are non-chiral hence products will be non-chiral and only 1 product will be formed in each case.
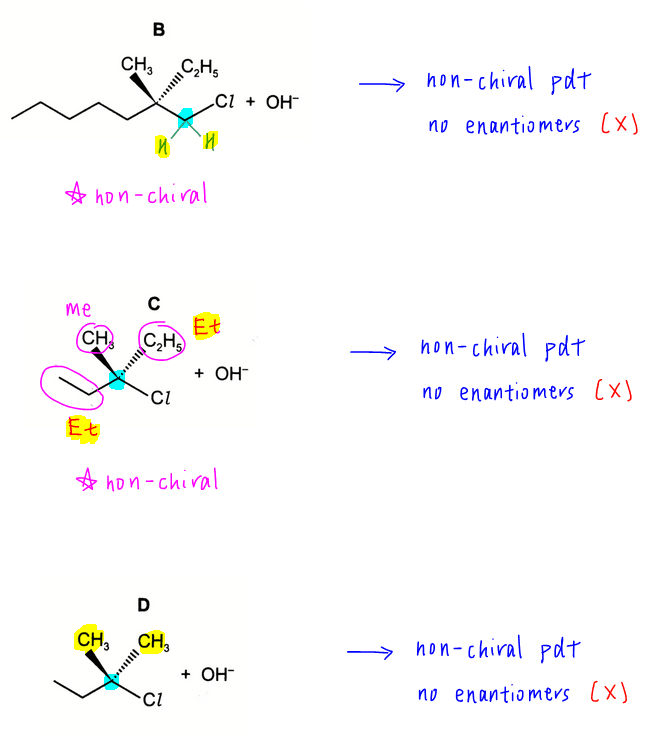
So therefore the answer to this question has to be A.
Check out this video for the full solution and detailed discussion!
Just a personal opinion the question would be a lot more challenging (and interesting) if one of the options from B to D involves a chiral halogenoalkane.
We would then need to deduce whether the mechanism is via SN1 or SN2, determine the stereochemistry of the product and compare with option A to see which is the best answer.
Topic: Nucleophilic Substitution and Nucleophilic Addition Mechanism, Organic Chemistry, A Level Chemistry, Singapore
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
You might also be interested in the suggested solution for Paper 1 Question 18.
Check out other A Level Chemistry Video Lessons here!
Looking for H2 Chemistry Tuition? Do consider taking up my classes at Bishan or online classes!
