A Level Chemistry 2017 Paper 1 Question 20 Solution - Exclusive
In this exclusive video we want to discuss the suggested solution for A Levels Chemistry (H2 Chemistry) 2017 Paper 1 Question 20.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this question:
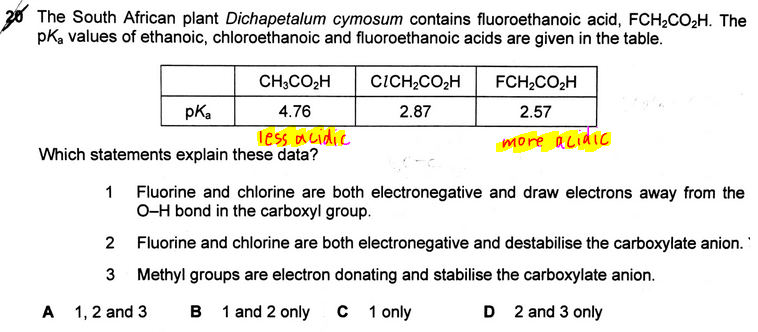
The topic tested in this question is on the acidity of carboxylic acids.
From the pKa values we know that the acidity of the acids are in the following order:
(least acidic) CH3COOH < ClCH2COOH < FCH2COOH (most acidic)
Acidity of an organic compound is related to the stablity of its conjugate base.
The more stable the conjugate base, the more likely the dissociation of the weak acid to form the conjugate base, the more H+ is released hence the more acidic the weak acid.
For chloroethanoic acid, the electronegative Cl group is electron withdrawing which helps disperse the negative charge on oxygen of the carboxylate anion.
The conjugate base is more stable therefore chloroethanoic acid is more acidic and has a smaller pKa value than ethanoic acid.
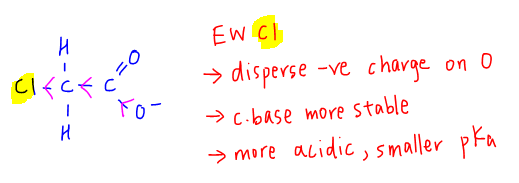
For fluoroethanoic acid, F is more electronegative than Cl which makes it a more electron withdrawing group.
The conjugate base will be stabilised to the greatest extent therefore fluoroethanoic acid is the most acidic and has the smallest pKa value.

Once we have the concept sorted out we can go through the 3 statements and decide that only statement 1 is valid. Hence the answer to this question is option C.

Check out this video for the full solution and detailed explanation!
Topic: Carboxylic Acids, Organic Chemistry, A Level Chemistry, Singapore
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
You might also be interested in the suggested solution for Paper 1 Question 19.
Check out other A Level Chemistry Video Lessons here!
Looking for H2 Chemistry Tuition? Do consider taking up my classes at Bishan or online classes!
