A Level Chemistry 2017 Paper 1 Question 25 Solution - Exclusive
In this exclusive video we want to discuss the suggested solution for A Levels Chemistry (H2 Chemistry) 2017 Paper 1 Question 25.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this question:
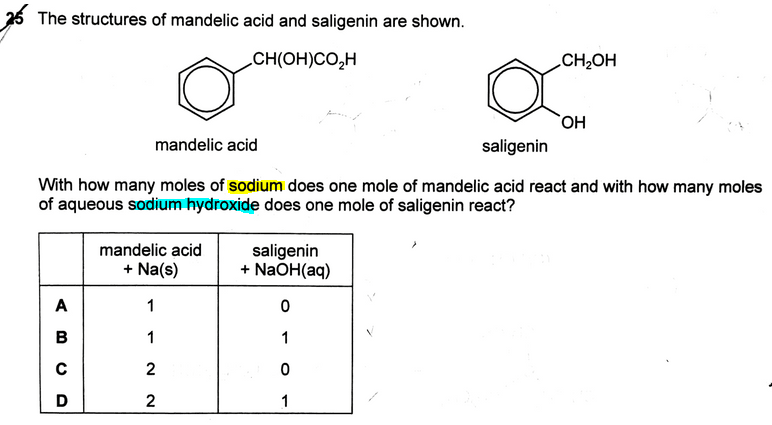
The topic tested in this question is on the acidity of organic compounds carboxylic acid, phenol and alcohol.
The acidity and reactions of these compounds with bases are as summarised in the table below:
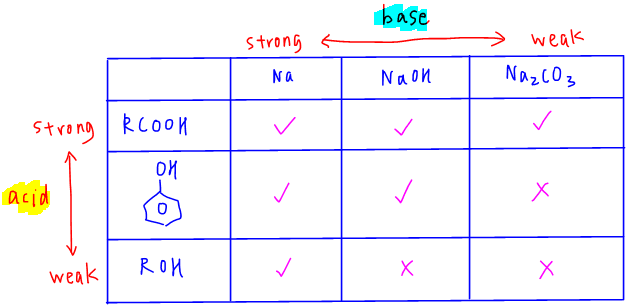
From the table we know that:
- carboxylic acid is the strongest acid and can react with Na, NaOH and Na2CO3
- phenol is less acidic and can react with Na and NaOH only
- alcohol is the least acidic and can only react with Na
We must always remember that alcohol is a weaker acid than water so therefore alcohols are neutral and cannot react with bases like NaOH and Na2CO3.
So we can now conclude that mandelic acid will react with 2 moles of Na due to one carboxylic acid and alcohol functional group.

Saligenin will react with 1 mole of NaOH due to one phenol functional group. Remember alcohol will not react with NaOH since it is neutral.
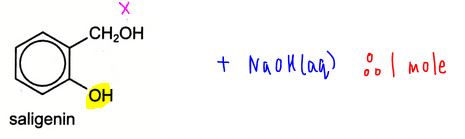
Finally we can compare the options and decide that the answer to this question is D.

Check out this video for the full solution and detailed explanation!
Topic: Acidity of Organic Compounds, Organic Chemistry, A Level Chemistry, Singapore
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
You might also be interested in this video on A Level Chemistry 2017 Paper 1 Question 24 Solution.
Check out other A Level Chemistry Video Lessons here!
Looking for H2 Chemistry Tuition? Do consider taking up my classes at Bishan or online classes!
