A Level Chemistry 2017 Paper 1 Question 5 Solution
In this video we want to discuss 2017 A Level H2 Chemistry Paper 1 Question 5.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through the question.
Topic tested is on Gaseous State.
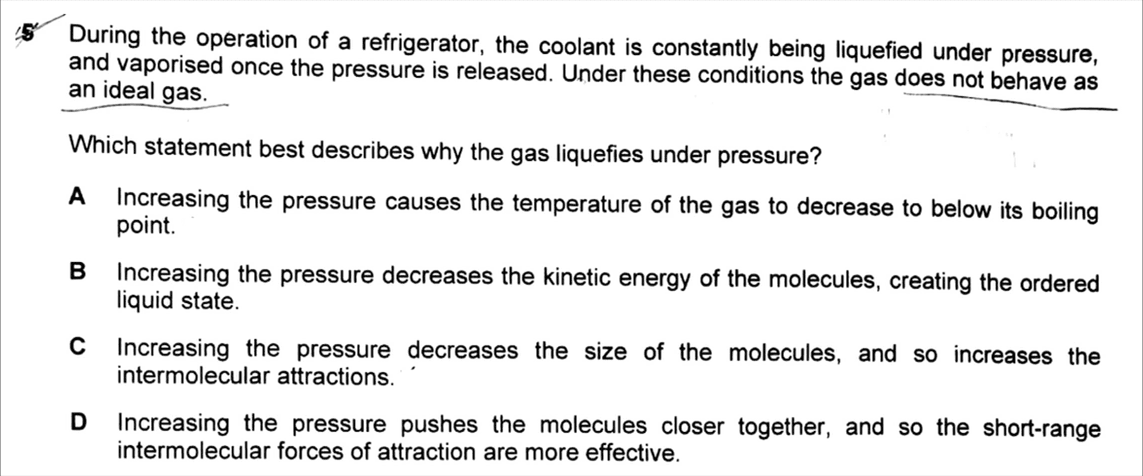
For this question we want to use the Ideal Gas Equation to explain why a gas will liquefy under high pressure.
The most important formula in Gasesous State is the Ideal Gas Equation PV = nRT where:
P is pressure in Pa,
V is volume in m3,
n is moles of gas,
R is gas constant 8.31 J K-1 mol-1, and
T is temperature in K.
Using this equation we can predict changes in state of a gas when another state changes.
For example:
1. At constant temperature, pressure will increase when volume decrease (Boyle's Law)
2. At constant pressure, volume will increase when temperature increase (Charles' Law).
Option A

When pressure increase the temperature should also increase based on the Ideal Gas Equation so option A is not true.
Option B

When pressure increase, temperature increases which means the kinetic energy of molecules should also increase since KE is directly related to temperature.
Therefore option B is not true.
Option C

Increasing pressure will bring the molecules closer together but cannot change the size of the molecules.
Therefore option C is not true.
Option D

Increasing pressure will bring the molecules closer together so intermolecular forces get more effective and stronger.
This statement makes the most sense hence option D is our best answer.
Check out this video for the question and the suggested solution!
Topic: Gaseous State, Physical Chemistry, A Level Chemistry, Singapore
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
You might also be interested in this video on A Level Chemistry 2017 Paper 1 Question 4 Solution.
Check out other A Level Chemistry Video Lessons here!
Looking for H2 Chemistry Tuition? Do consider taking up my classes at Bishan or online classes!
