A Level Chemistry 2017 Paper 1 Question 6 Solution - Exclusive
In this exclusive video we want to discuss the suggested solution for A Levels Chemistry (H2 Chemistry) 2017 Paper 1 Question 6.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this question.

Topic tested is on Ionic Equilibria and definitions of Bronsted-Lowry Acid and Base, and Lewis Acid and Base.
Bronsted-Lowry Theory of Acids and Bases is based on proton transfer:
A Bronsted acid is defined as a proton or H+ donor, while
A Bronsted base is a proton or H+ acceptor.
Lewis Theory of Acids and Bases is based on electron pair transfer:
A Lewis acid is an electron pair acceptor, while
A Lewis base is an electron pair donor.
So based on these definitions we can compare the options and see which of these species can function as both a Lewis base and Bronsted-Lowry acid.
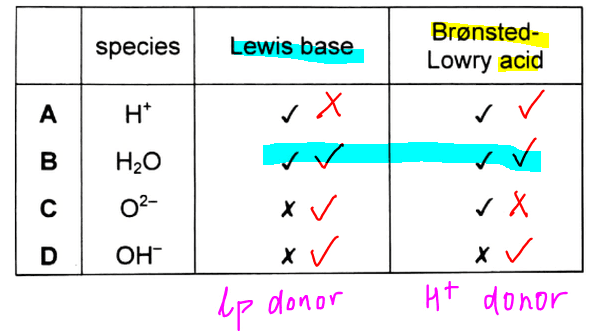
Therefore B is the best answer as H2O can donate lone pair to form H3O+ (Lewis base) and it also also donate H+ to form OH- (Bronsted acid).
Check out this video for the question and the suggested solution!
Topic: Ionic Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
You might also be interested in this video on A Level Chemistry 2017 Paper 1 Question 5 Solution.
Check out other A Level Chemistry Video Lessons here!
Looking for H2 Chemistry Tuition? Do consider taking up my classes at Bishan or online classes!
