A Level Chemistry 2017 Paper 1 Question 7 Solution - Exclusive
In this exclusive video we want to discuss the suggested solution for A Levels Chemistry (H2 Chemistry) 2017 Paper 1 Question 7.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this question.

Topic tested is on Periodicity of Period 3 Elements, in particular the comparision of electronegativity and atomic radius between magnesium, aluminium, silicon and phosphorus.
The atomic radius can easily be determined from the Data Booklet and we can find that atomic radius decreases from magnesium to phosphorus.
Electronegativity is deduced from the knowledge that fluorine, a Group 17 element, is the most electronegative element.
So the closer the element is to fluorine, the more electronegative it will be.
Therefore phosphorus, which is in Group 15, is closer to Group 17 therefore is more electronegative than magnesium, a Group 2 element.
Putting these two concepts together, the trends and expected graph of electronegativity against atomic radius should look something like this.
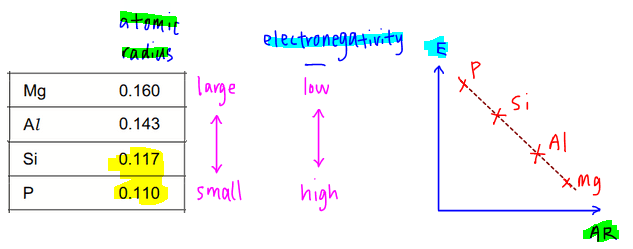
So with the trend of atomic radius and electronegativity determined, we can compare the options in the question and choose our best answer.
Out of the 4 options, A and C are eliminated straight away since the shape of the graph is not as expected.
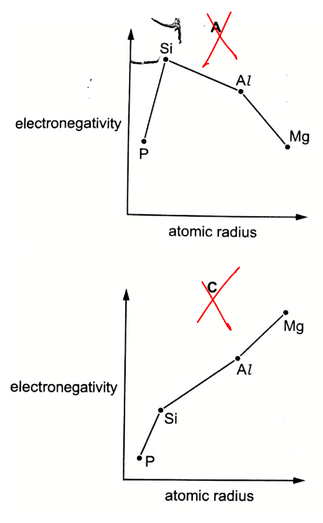
Between options B and D, B is the better answer since there is only a small difference in atomic radius between P (0.110 nm) and Si (0.117 nm).

Check out this video for the full discussion and suggested solution!
Topic: Periodicity, Inorganic Chemistry, A Level Chemistry, Singapore
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
You might also be interested in this video on A Level Chemistry 2017 Paper 1 Question 6 Solution.
Check out other A Level Chemistry Video Lessons here!
Looking for H2 Chemistry Tuition? Do consider taking up my classes at Bishan or online classes!
