Alcohol Reactions, Phenol Reactions - Organic Chemistry
In this video we go through the 7 Must-Know Reactions of Alcohols and 5 Must-Know Reactions of Phenols.
Must-Know Alcohol Reaction
1. Elimination or dehydration of alcohol to form alkene using concentrated sulphuric acid at 170 degree celsius or aluminium oxide, heat.

2. Nucleophilic substitution of alcohol to form chloroalkane using anhydrous PCl5 or SOCl2 at room temperature

3. Nucleophilic substitution of alcohol to form bromoalkane using PBr3 or HBr, warm

4. Oxidation of primary alcohol to form carboxylic acid using KMnO4 or K2Cr2O7 in dilute H2SO4, heat or reflux. Secondary alcohols will be oxidised to ketones under these reagents and conditions.
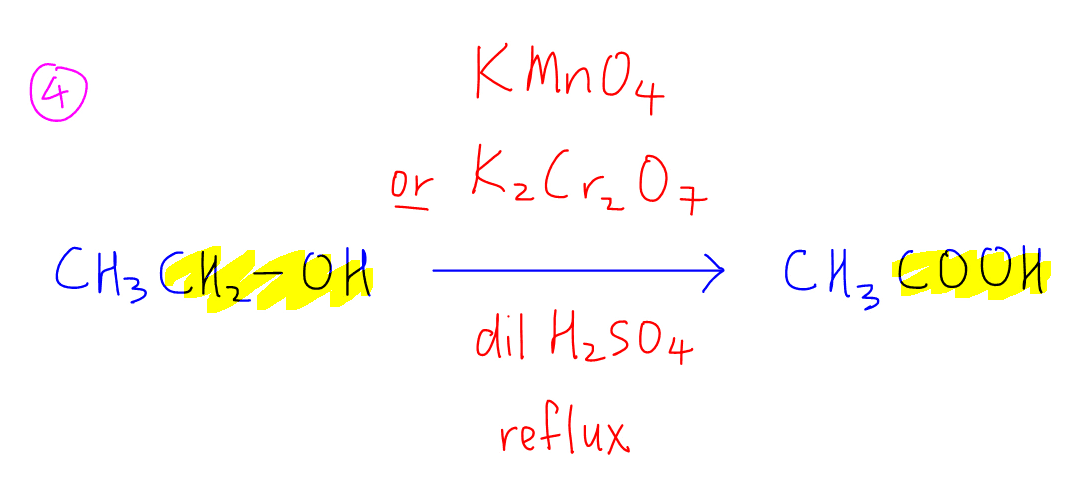
5. Mild oxidation of primary alcohol to form aldehyde using K2Cr2O7 in dilute H2SO4, reflux with immediate distillation

6. Neutralisation of alcohol to form alkoxide using sodium metal at room temperature

7. Tri-iodomethane test of some alcohols with iodine in aqueous sodium hydroxide, warm, to give yellow precipitate

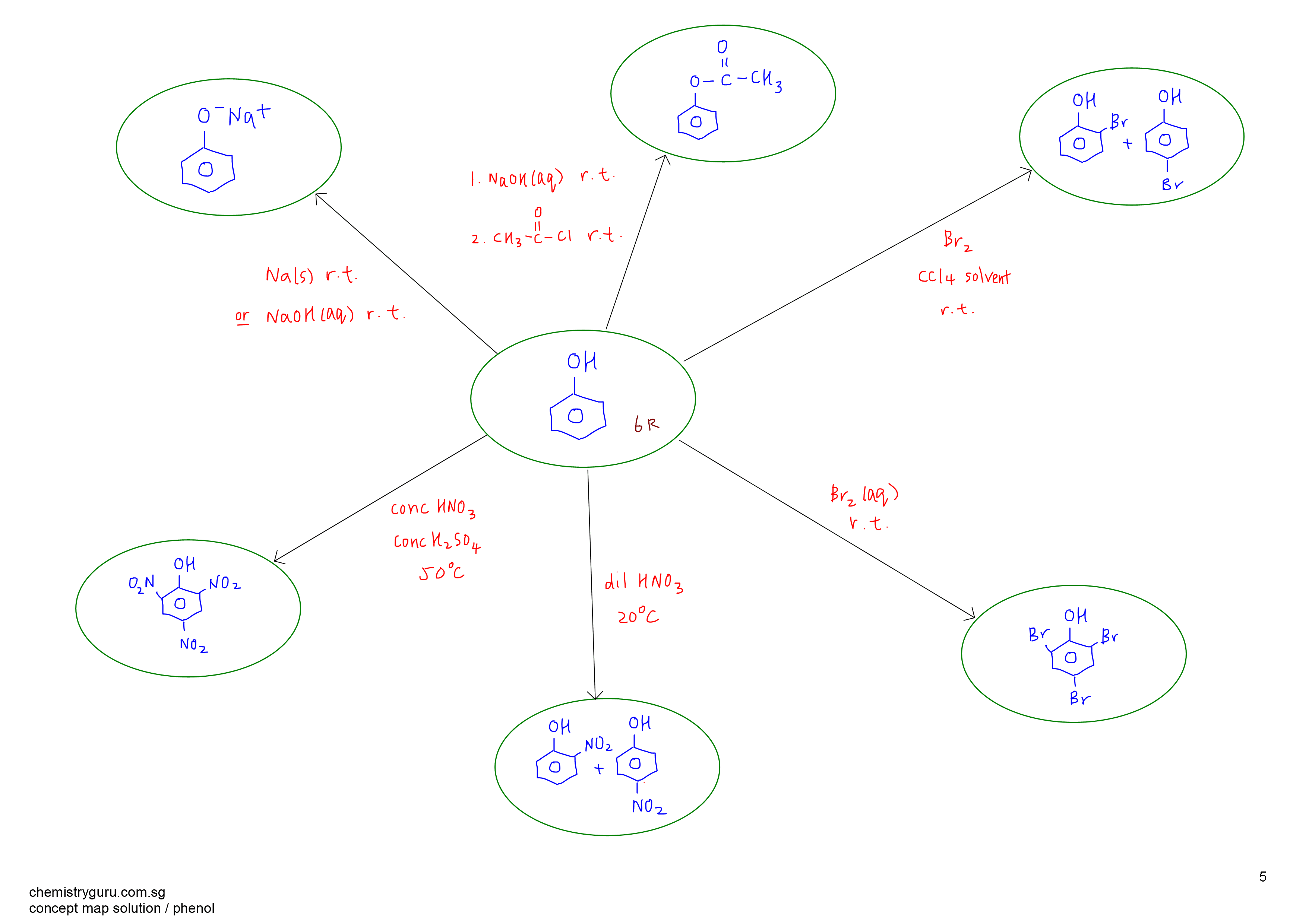
Must-Know Phenol Reaction
1. Neutralisation of phenol to form phenoxide using sodium metal or aqueous sodium hydroxide at room temperature

2. Electrophilic substitution of phenol with bromine in organic solvent at room temperature to form 2-bromophenol and 4-bromophenol

3. Electrophilic substitution of phenol with aqueous bromine at room temperature to form 2,4,6-tribromophenol

4. Electrophilic substitution of phenol with dilute nitric acid at 20 degree celsius to form 2-nitrophenol and 4-nitrophenol

5. Electrophilic substitution of phenol with concentrated nitric acid in concentrated sulphuric acid at 50 degree celsius to form 2,4,6-trinitrophenol

Memorising the reagents and conditions for these alcohol reactions is an integral part of Organic Chemistry, so I've added 2 short memory tests.
Let's see if you can remember all of them!
Alcohol Concept Map

Phenol Concept Map
Download free Organic Chemistry concept maps here!
Topic: Alcohols, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes!
