Determine Major Product for Alkene Bromination in conc NaCl
Let Chemistry Guru, Singapore's top choice for JC Chemistry tuition, guide you through this week's question.
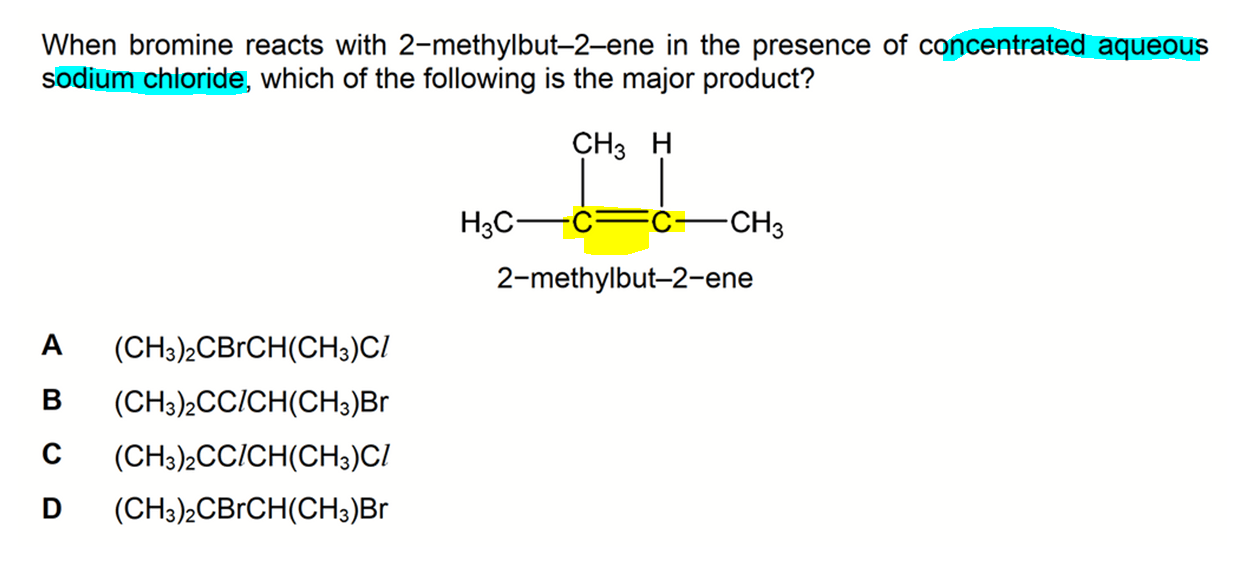
We need to determine the major product when 2-methylbut-2-ene reacts with bromine in the presence of concentrated NaCl(aq).
Notice 2-methylbut-2-ene is asymmetrical so there will be a mixture of products.
Also reacting with bromine in the presence of conc NaCl(aq) is not in syllabus so we have to deduce the products based on the electrophilic addition mechanism of alkene.
Check out this video for a detailed discussion of alkene electrophilic addition mechanism.
1. Deduce Electrophile in Step 1
First let us deduce the electrophile that reacts with alkene in the first step.
The available species in bromine in conc NaCl(aq) are Br2, H2O and Cl-. Na+ is stable so it will not act as electrophile.
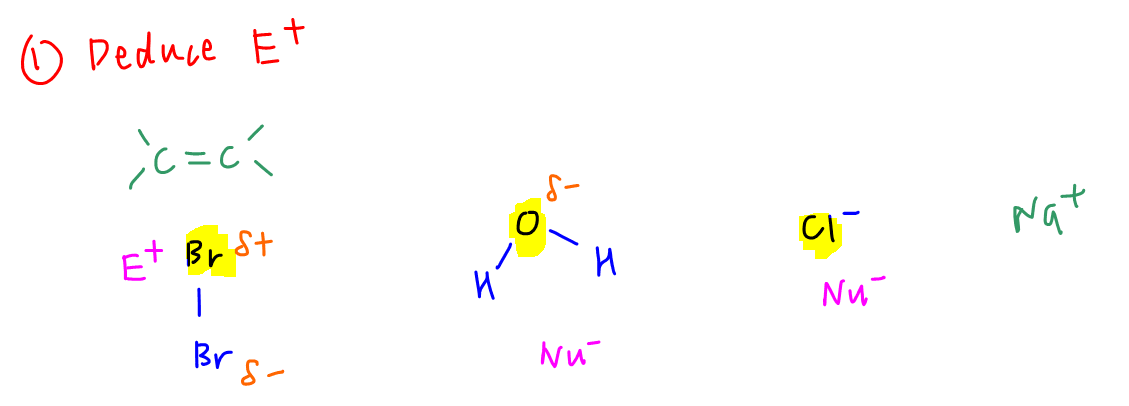
Out of the 3 possible species, only Br2 can function as electrophile due to partial positive charge on one of the bromine atom.
Oxygen in water is partial negative and chloride ion is negative hence cannot be electrophilic.
2. Determine More Stable Carbocation
Remember 2-methylbut-2-ene is asymmetrical so depending on which alkene carbon attacks electrophilic bromine, there will be 2 possible carbocations.
Let's draw the first step of electrophilic addition mechanism to determine the 2 carbocations formed.
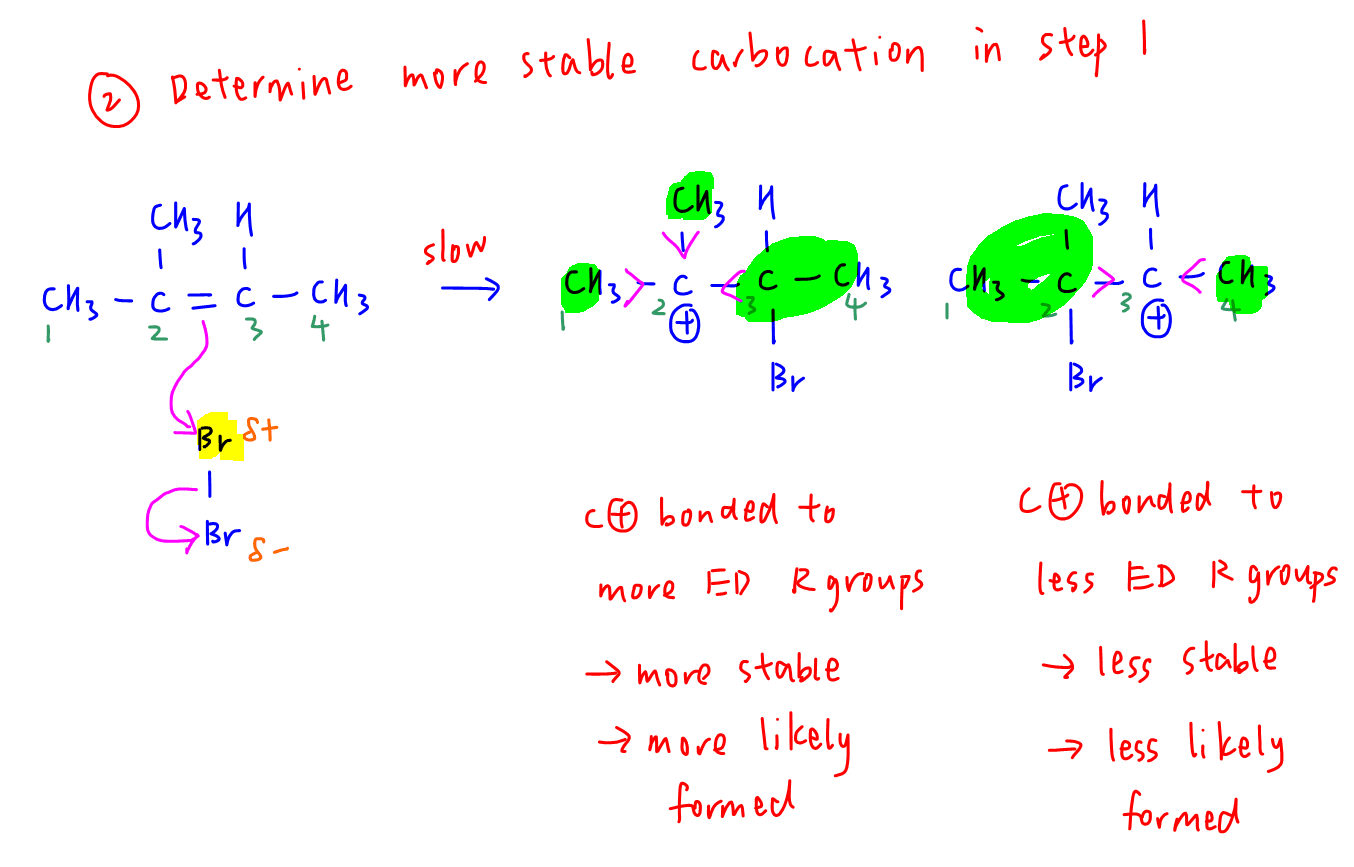
When the carbocation is on carbon 2, it is attached to more electron donating alkyl groups which disperse the positive charge on carbocation to a greater extent.
This makes the carbocation more stable and it is more likely formed as compared to carbocation on carbon 3.
3. Determine Nucleophile in Step 2
We can now focus on the available nucleophiles and deduce which is most likely to react with the more stable carbocation in the second step.
Available nucleophiles are Br- (formed from first step), OH- (from water), Cl- (from conc NaCl).
Since Cl- is negatively charged and concentration is high, it is the most likely nucleophile to react in second step.

Therefore the answer to this question will be option B (3-bromo-2-chloro-2-methylbutane)
Topic: Alkenes, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's esteemed H2 Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
