Back Titration
When we need to determine the amount of an unknown substance, we usually can use direct titration with a reactant.
Sometimes the reaction involves solids or gaseous products, so direct titration is not feasible or difficult to measure.
We can then use back titration to determine the amount of substance, where an excess known amount of reagent is reacted with this substance, then the remaining amount of reagent is determined with another reaction via titration.
Let's use an example to illustrate this.
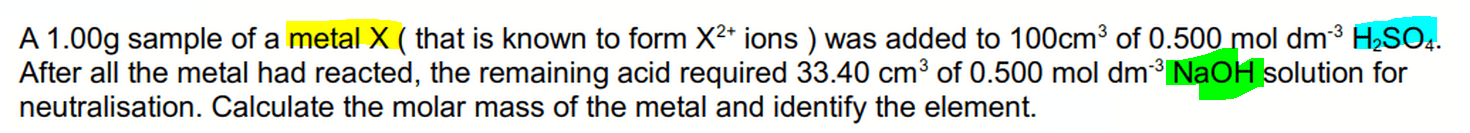
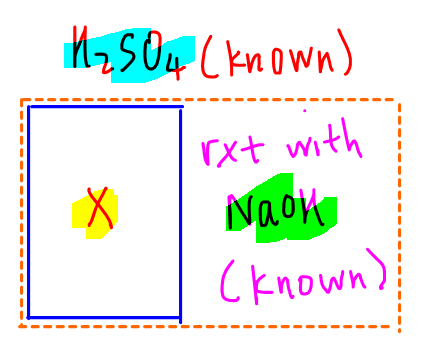
To better visualise the process, students are strongly encouraged to draw the experimental diagram and fill in all the information such as volume and concentration.
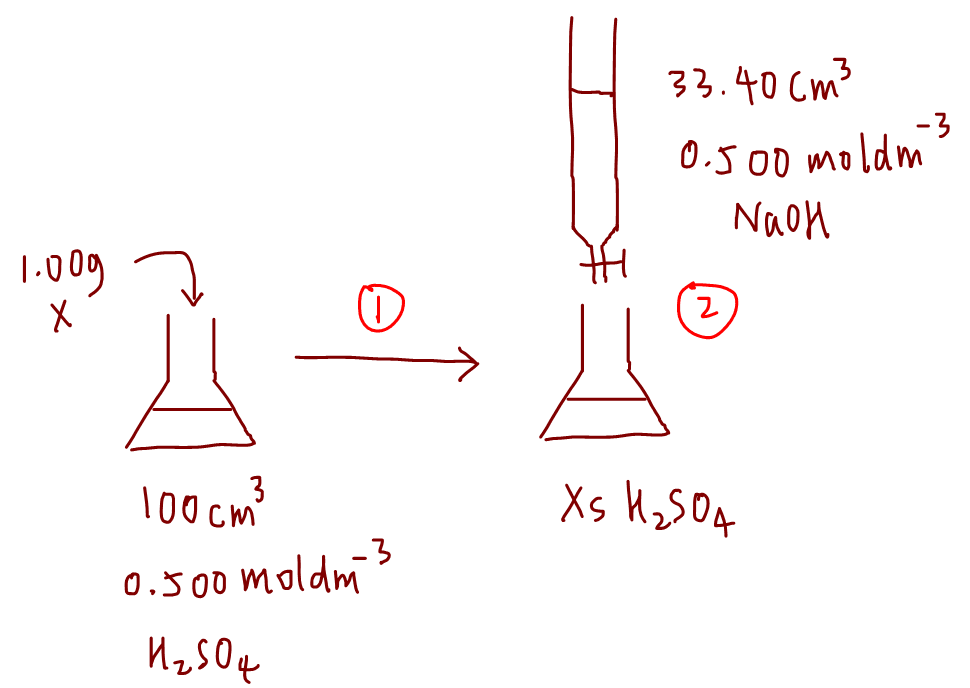
This way we can refer to the diagram instead of the question when we do the calculations.
We also need the balanced equations for all reactions taking place so that we can use the mole ratios for calculation.
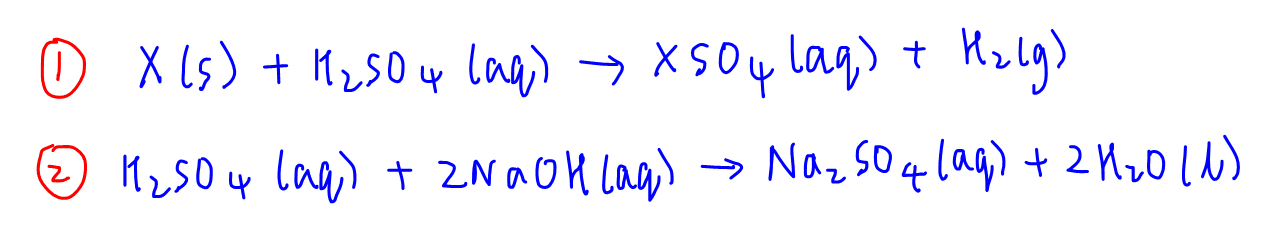
From the diagram, we can start with the titration first and determine the amount of NaOH used.
Then using mole ratio from reaction 2, we can work backwards (hence the name back titration) and determine the amount of H2SO4 that reacts with NaOH.
We can also calculate the total amount of H2SO4 used.
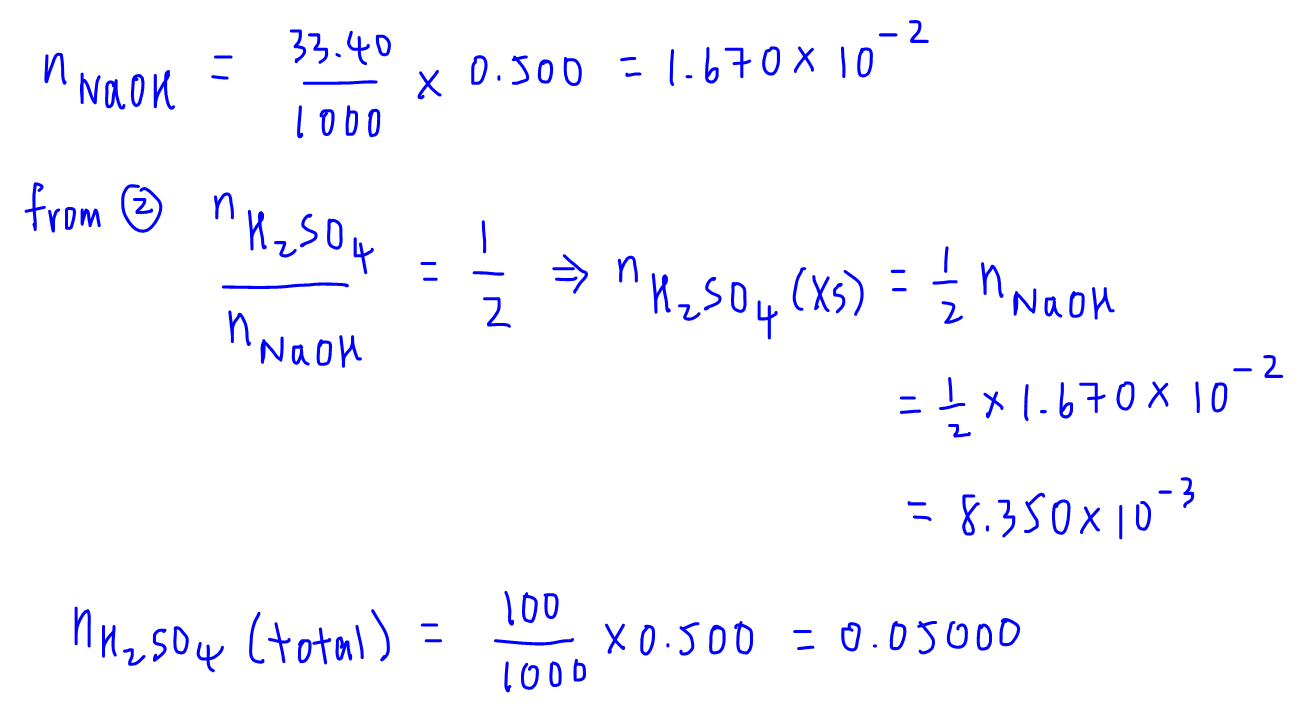
We know that part of the total amount of H2SO4 added has reacted with X, and the remaining amount of H2SO4 reacted with NaOH.
This means that the amount of H2SO4 that reacts with X will be the total amount of H2SO4 less amount of H2SO4 that reacted with NaOH.
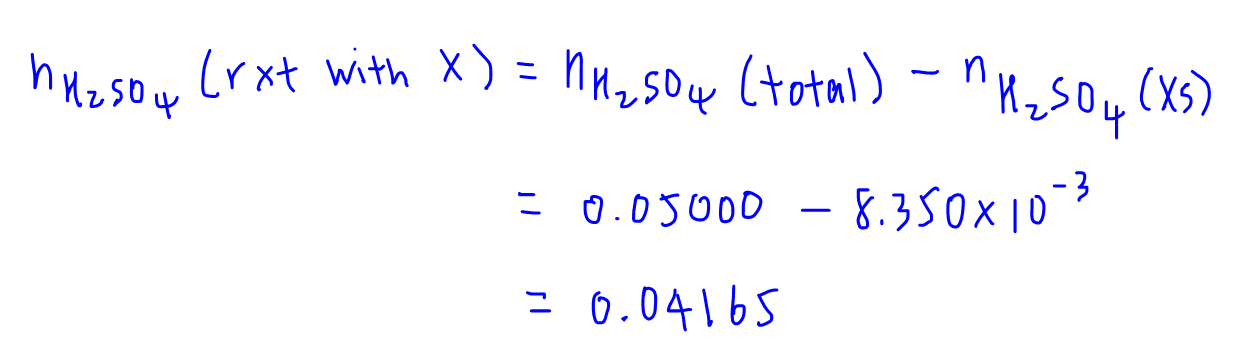
We can then use mole ratio from reaction 1 to determine amount and molar mass of X.

Using the answer 24.0 g mol-1 and Periodic Table, we can find the Group 2 metal with the closest mass number, ie Magnesium with relative atomic mass of 24.3.
For the detailed step-by-step discussion on how to answer back titration questions, check out this video!
Topic: Mole Concept, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online chemistry classes!
