2019 A Level H2 Chemistry Paper 1 Question 4 - Deduce Dot-Cross Diagram for Barium Peroxide
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through Paper 1 Question 4.
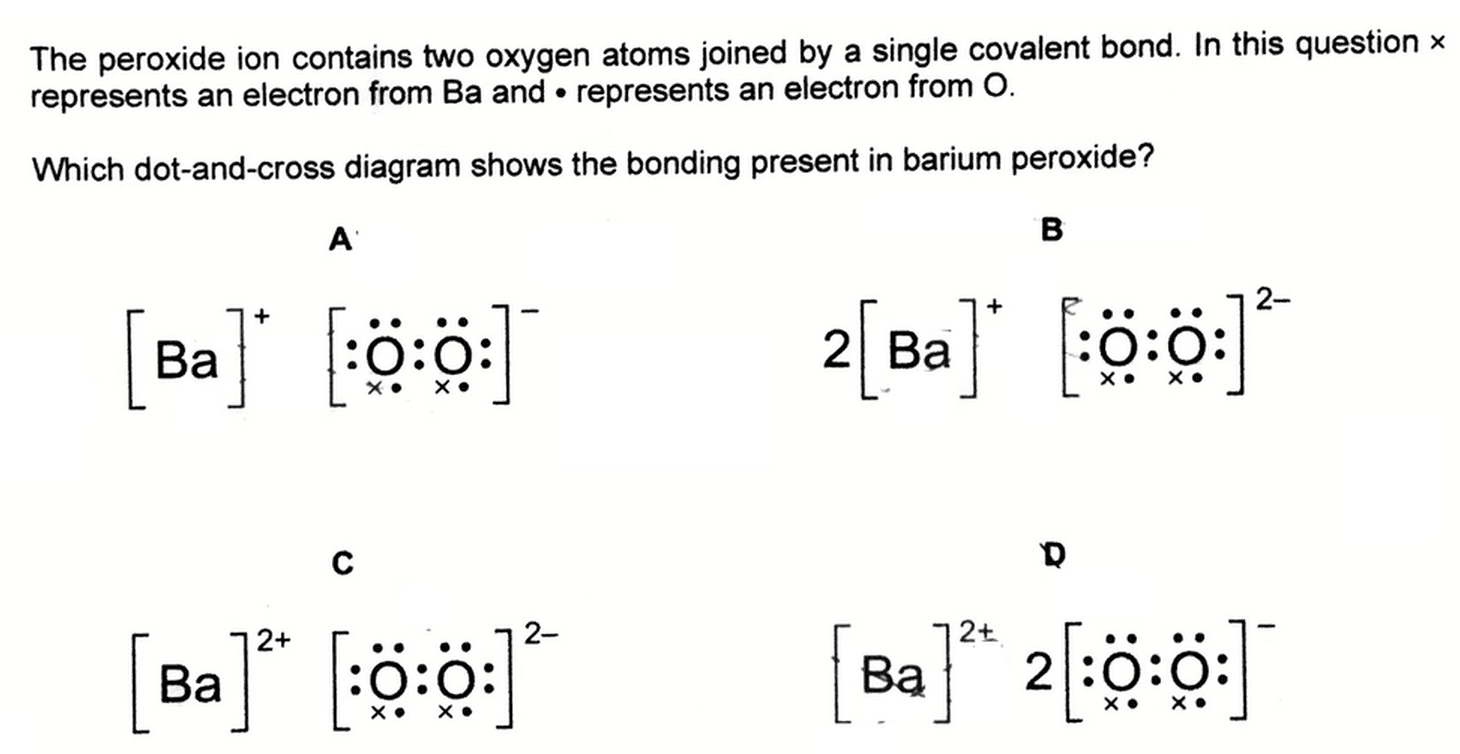
We need to deduce the dot and cross diagram for barium peroxide.
1. Deduce barium cation
Since barium is a Group 2 metal, it will lose 2 electrons to form Ba2+ cation.
Hence options A and B where Ba has a +1 charge will be eliminated.
2. Deduce peroxide anion
For both options C and D, each oxygen atom has an additional electron (x).
This means peroxide will gain a total of 2 additional electrons and its charge should be a -2.
We can also deduce this from hydrogen peroxide H2O2.
To form peroxide from hydrogen peroxide, we can treat H2O2 as a diprotic acid which donates 2 protons.
Both O-H bonds are broken to release 2 H+, hence the peroxide will have an overall charge of -2.
Therefore the answer to this question will be C.
Topic: Chemical Bonding, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2019 A Level H2 Chemistry Paper 1
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
