Basicity of Acids and Dissociation of Diprotic Acids
In this JC2 webinar we want to explain basicity of acids and learn how to interprete the dissociation of diprotic acids.
1. Basicity of Acids
Basicity of acids is related to the number of protons an acid can donate.
A monoprotic acid donates 1 proton or 1 H+, eg strong acid HCl and weak acid CH3COOH
A diprotic acid donates 2 protons or 2 H+, eg strong acid H2SO4 and weak acid H2CO3
Similarly, a monobasic base accepts 1 proton and a dibasic base accepts 2 protons.
2. Dissociation of Diprotic Acid H2CO3
A diprotic acid will not donate both of its protons in 1 reaction.
The dissociation will occur in 2 stages:
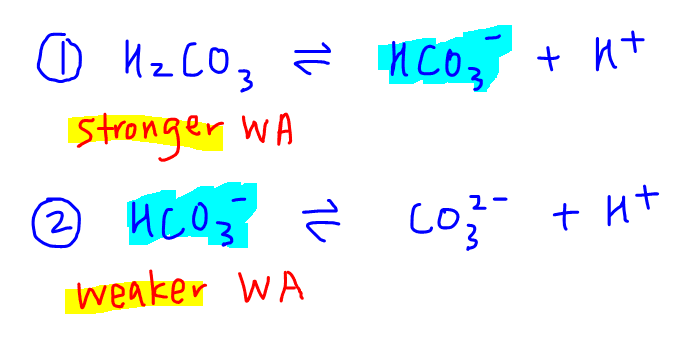
Notice each step now becomes the dissociation of a monoprotic acid.
The acid in the first dissociation is H2CO3, while the acid in the second dissociation is HCO3-.
This means we need to be very familiar with the concept and dissociation of monoprotic acids, and apply them to diprotic or polyprotic species.
3. Comparing Acid Strength
For a polyprotic acid, the first acid is always the strongest and the strength decreases as the dissociation proceeds.
This means that the first acid is always stronger than the second acid, which is in turn stronger than the third acid (if triprotic).
For the H2CO3 example given earlier, H2CO3 is a stronger acid than HCO3-.
H2CO3 has more protons to donate than HCO3-, while HCO3- is negatively charged so it's harder to lose a positive H+.
Hence H2CO3 is a stronger acid than HCO3-.
For a polyprotic base, the trend is the same.
The first base is always stronger than the second base which is in turn stronger than the third base (for triprotic bases).
Understanding how we handle polyprotic acids and bases as a series of monoprotic species will be useful for subsequent concepts discussed in Ionic Equilibria, eg determine pH of diprotic acids.
Topic: Ionic Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
