Comparing Basicity of Imidazole, Phenylamine and Amide
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to compare basicity of 3 nitrogen compounds - imidazole, phenylamine and amide.
We should be familiar with basicity of phenylamine and amide, but imidazole is a new compound that we would need some time to understand and explain.
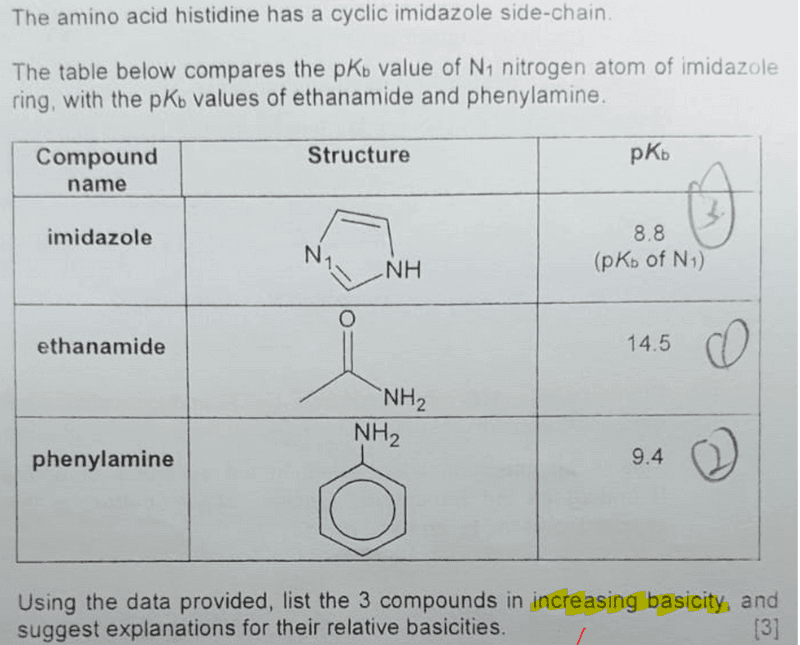
1. List the 3 compounds in increasing basicity
A stronger base will dissociate to a greater extent to give more OH-, hence its Kb will be bigger, and pKb smaller.
So listing down the 3 compounds from weak basicity (large pKb) to strong basicity (small pKb) is pretty straightforward:
ethanamide < phenylamine < imidazole
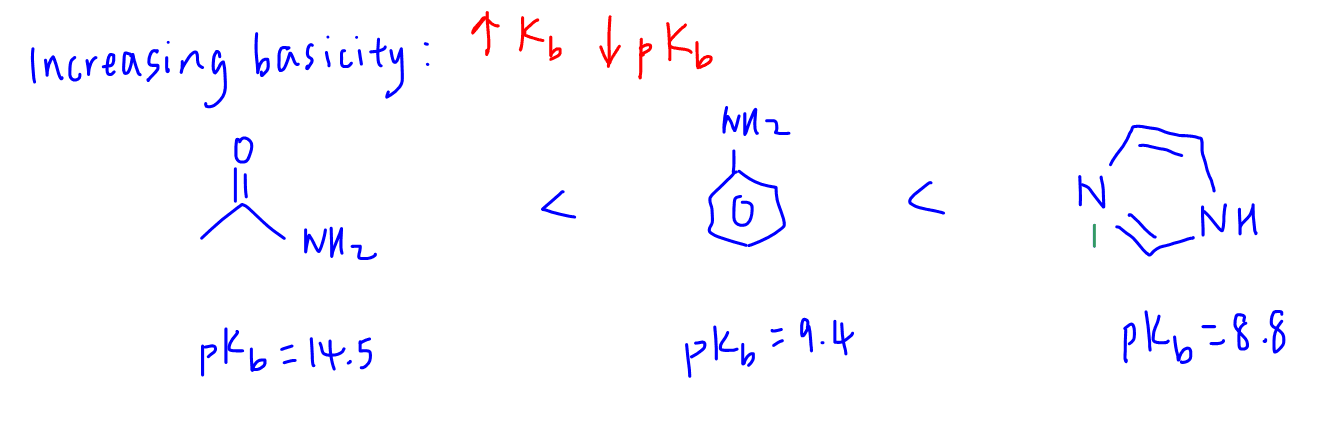
2. Explain why amide is least basic (neutral)
The lone pair on nitrogen is delocalised extensively between 2 electronegative nitrogen and oxygen.
Hence it is not available for donation.
This means amide is neutral.
We can draw the resonance structures to show the delocalisation of lone pair between N to O.
3. Explain why phenylamine is basic, but less basic than ammonia
The lone pair on nitrogen interacts with the delocalised pi system of benzene.

Therefore it is less available for donation which makes phenylamine a weaker base than NH3.
But phenylamine is still able to function as a base, hence it is more basic and has a lower pKb than amide.
For comparing basicity of nitrogen compounds amine, ammonia, phenylamine and amide, check out my previous video.
4. Explain why N1 of imidazole is most basic
There are 2 nitrogens in imidazole (let's name them N1 and N4).
Interestingly both nitrogens seem to have lone pairs but N1 is basic while N4 is neutral.

Although not very obviously mentioned in the question, we can deduce that the cyclic structure in imidazole is aromatic.
In the cyclic structure, there are 2 pi bonds (between C2-C3 and N1-C5) and one lone pair on N4 that can interact with each other to form a delocalised pi system.
This delocalisation is interesting because the number of delocalised electrons is 6, which is the same as that of benzene.
This means that like bezene, imidazole has a closed delocalised ring of 6 pi electrons.
Therefore it is considered an aromatic compound with resonance stability, just like benzene.
Since N4 needs to use its lone pair for aromaticity, it is not available for donation and N4 is neutral.
N1 on the other hand already forms a pi bond which contributes to the delocalised system.
N1 is sp2 hybridised and its basic shape is trigonal planar.
Its lone pair is pointing away from the cyclic structure and is unable to interact with the delocalised pi system.
Therefore the lone pair will be available for donation, which makes N1 the strongest base in this question.
Topic: Nitrogen Compounds, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
