How to Draw Born Haber Cycle of Ionic Compounds - Energetics
In this video we want to learn how to draw the Born Haber Cycle of ionic compounds using CaCl2 as an example.
We usually use the energy level diagram to present the Born Haber Cycle, which can be broken down into 4 steps.
1. Formation
Formation involves the reaction from elements in the standard state to ionic compound. Usually enthalpy change of formation for ionic compounds is exothermic as ionic compounds are stable.
We can draw this close to the bottom of the energy level diagram as the rest of the terms would be endothermic hence pointing upwards.

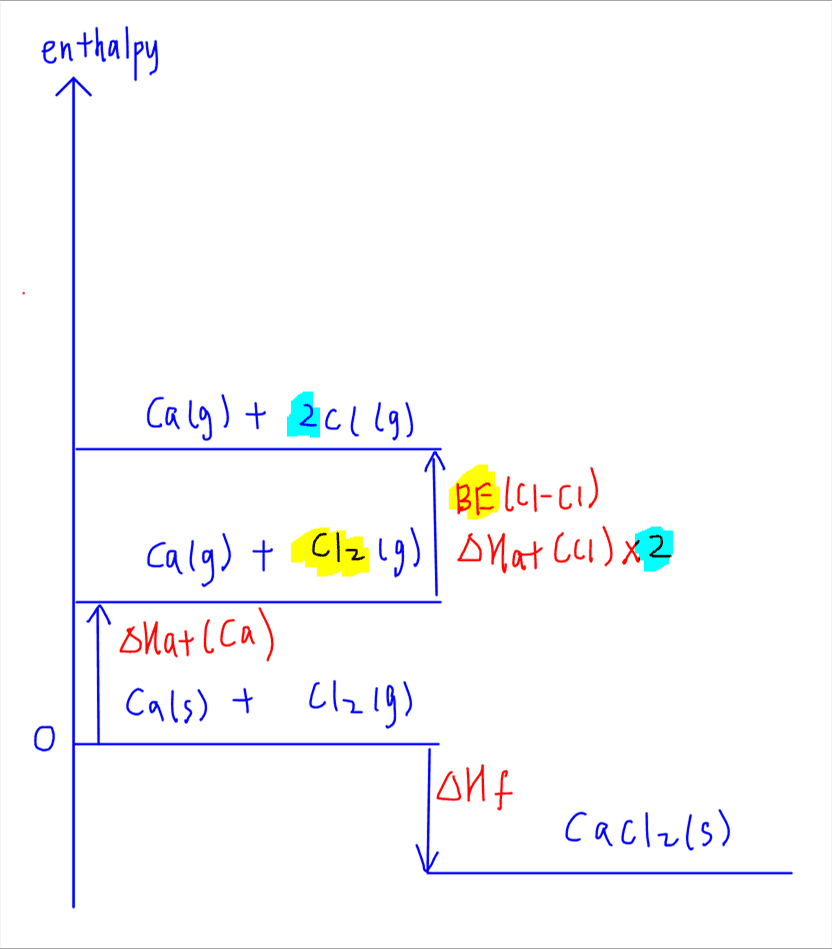
2. Atomisation
Atomisation involves forming gaseous atoms for both calcium metal and chlorine gas. Both terms are endothermic as energy is required to break all bonds in the elements to form gaseous atoms.
For Ca we will be given the enthalpy change of atomisation of metal.
For Cl2 usually we use bond energy of Cl-Cl bond which can be found in the Data Booklet.
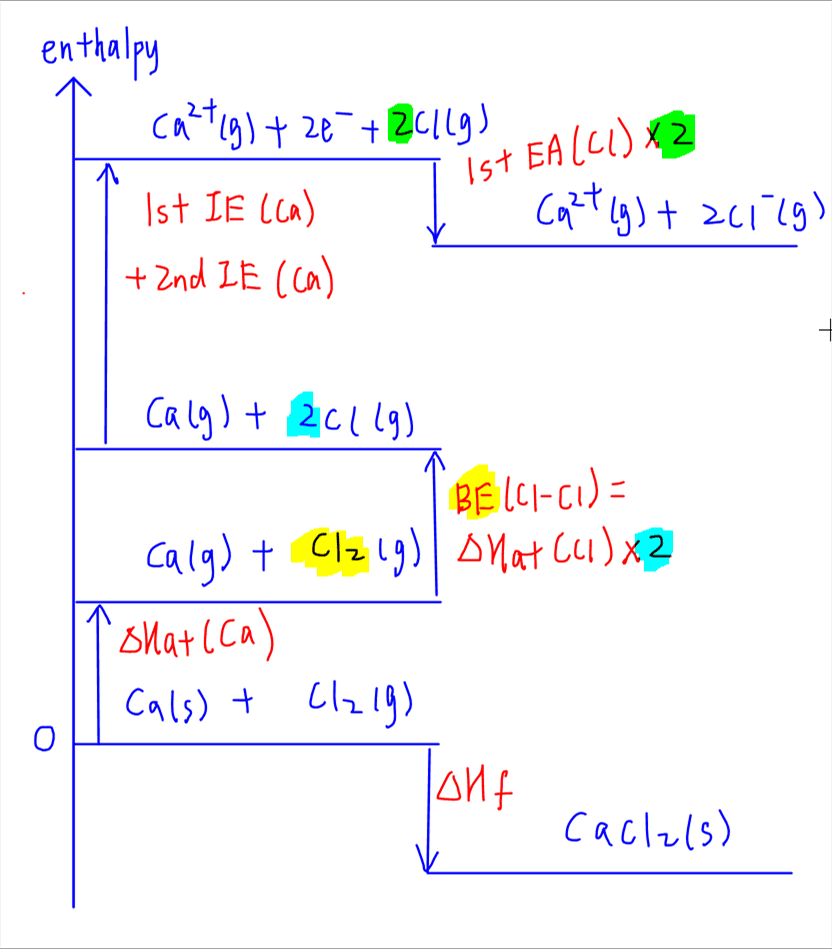
3. Ionisation
Ionisation involves removing the electrons from Ca (ionisation energy) and adding electrons to Cl (electron affinity).
Removing 2 electrons from Ca to form Ca2+ will be the first and second ionisation energies of Ca which can be found in the Data Booklet. Ionisation energy is endothermic as energy is required to overcome attraction between nucleus and valence electron.
Adding electron to Cl to form Cl- will be the first electron affinity of Cl. First electron affinity is exothermic as energy is released from attraction formed between nucleus and added electron.
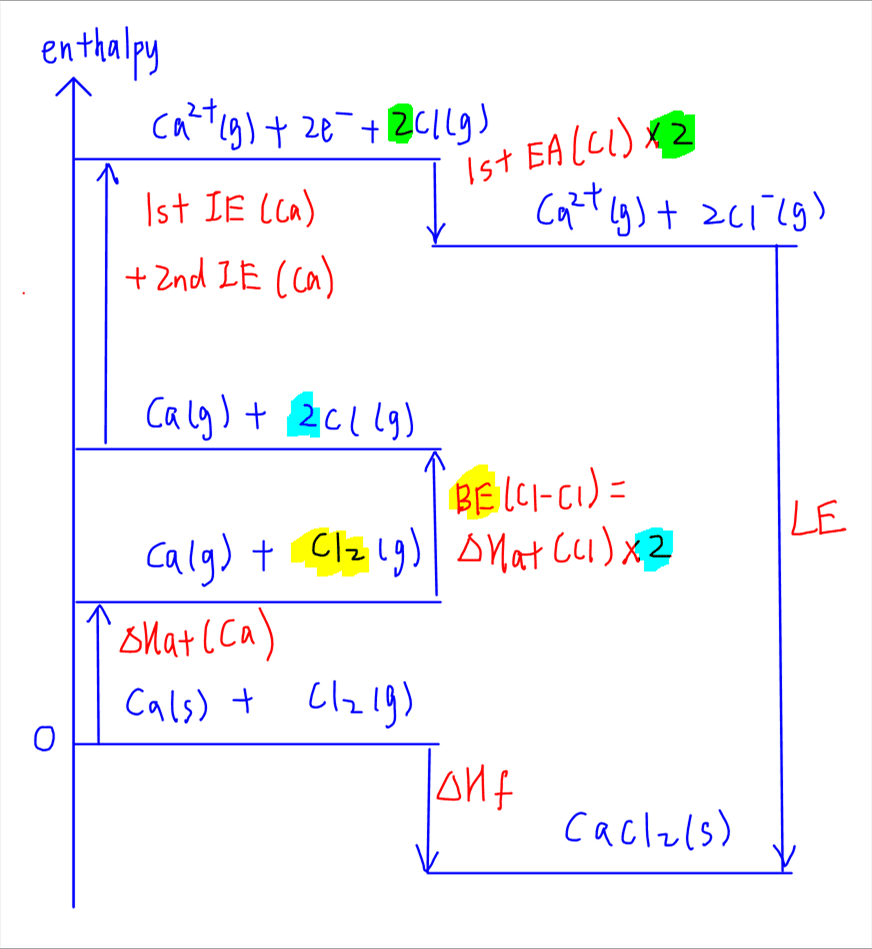
4. Lattice Energy
The last step involves lattice energy which is the forming of ionic compound from its constituent gaseous ions.
Since strong ionic bonds are formed, lattice energy is highly exothermic.
Hess' Law
We can use the follow expression to work out the relationship of all the terms in the Born Haber cycle. This expression is valid for all ionic compounds.
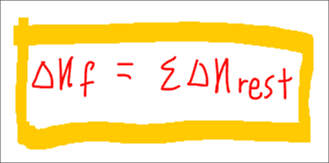
For the detailed step-by-step discussion on how to draw the Born Haber Cycle, check out this video!
Topic: Energetics, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online chemistry classes!
