Calculate Relative Atomic Mass from Isotopic Abundance
Each element will usually consist of a few different isotopes with the same proton number but different neutron number.
Therefore the mass number for these isotopes will be different and we call this the relative isotopic mass.
Each isotope will also have its own naturally occuring abundance which we refer to as isotopic abundance.
We can calculate the relative atomic mass of an element from the relative isotopic mass and abundance of its isotopes.
Let's use krypton as an example.
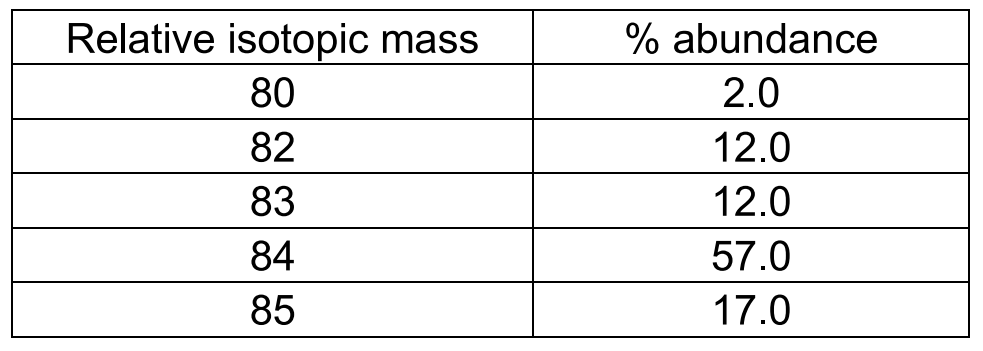
To determine relative atomic mass, we simply multiply each isotopic mass by its abundance, add all the values together and divide the total value by 100 percent.
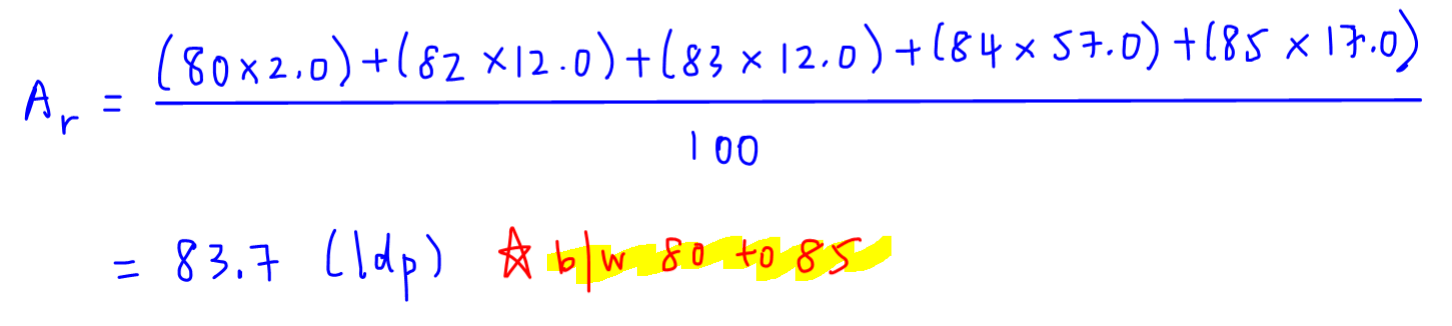
Effectively we are calculating the weighted average of the masses of isotopes, taking into account their relative abundances.
There are 2 things we can take note of to verify our answer.
1. The relative atomic mass must be between the isotopic mass of the smallest and biggest isotopes.
In this case our relative atomic mass of 83.7 is between 80 to 85.
2. The relative atomic mass must be closest to the isotope of biggest abundance.
In this case the relative atomic mass of 83.7 is closest to isotopic mass 84 with 57% abundance.
Topic: Mole Concept, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
