How to Calculate Percentage Composition by Mass
Let's learn how to calculate percentage composition by mass in this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre.
The percentage mass of an element X in a compound is equal to the percentage molar mass of X in that compound.

This means the mass ratio of X to compound is equal to molar mass ratio of X to compound.
To understand this, we can use water as an example.

One molecule of water is made up of 2 hydrogen atoms and 1 oxygen atom.
Since water is a compound made up of 2 or more elements chemically combined together in a fixed proportion, the mole ratio of hydrogen to oxygen will always be 2 to 1 regardless of the amount of water.
In 1 gram of water, the mole ratio of H to O is 2 to 1, and we can determine the mass ratio of H to O.
In 1kg of water, the mole ratio of H to O is still 2 to 1, therefore the mass ratio of H to O will remain the same.
In fact in any quantity of water, the mass ratio of H to O will be constant since mole ratio of H to O will always be 2 to 1.
We can peg this mass ratio to the molar mass ratio since we can determine molar mass from the periodic table.
For example to determine mass of oxygen in 10g of water, we can use the mass ratio equal to molar mass ratio formula to get our answer quickly.
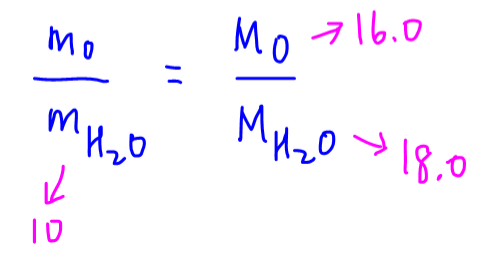
Topic: Mole Concept, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
