Calculate pH Change of Buffer when Hydroxide Added
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to learn how to calculate pH change of buffer when small amounts of hydroxide is added.
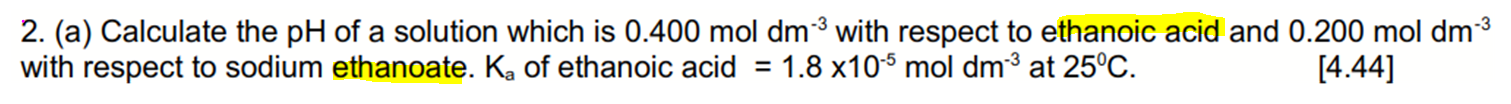
This solution is a mixture of weak acid ethanoic acid and conjugate base ethanoate.
Therefore this solution is a buffer solution.
Check out this video for an more indepth discussion of how to identify buffer solution.
Hence we can use the buffer equation to calculate its pH.
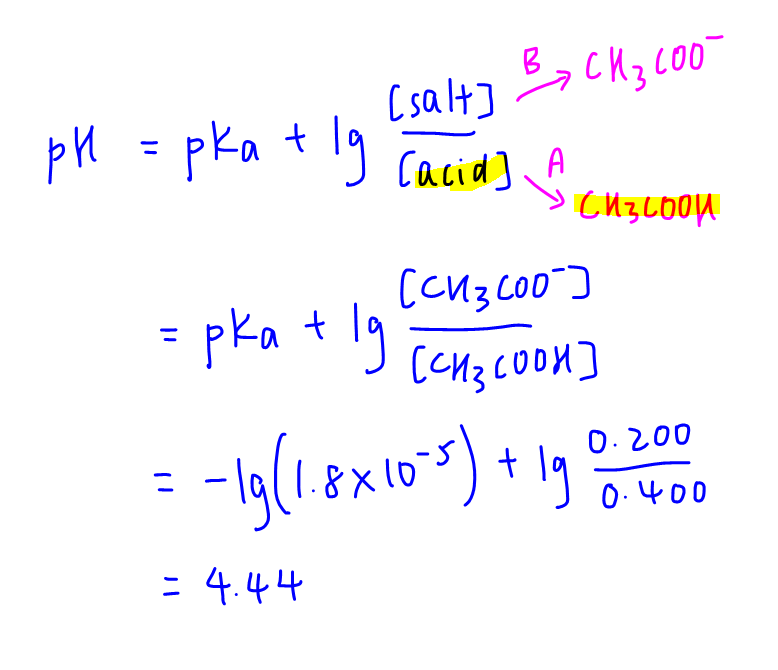
Learn how to calculate pH of buffer solution here.

Next 0.050 mol of sodium hydroxide is added to this solution.
Since the function of a buffer is to maintain pH, it must react away this added hydroxide.
Therefore it will be the job of ethanoic acid to remove this amount of OH-.
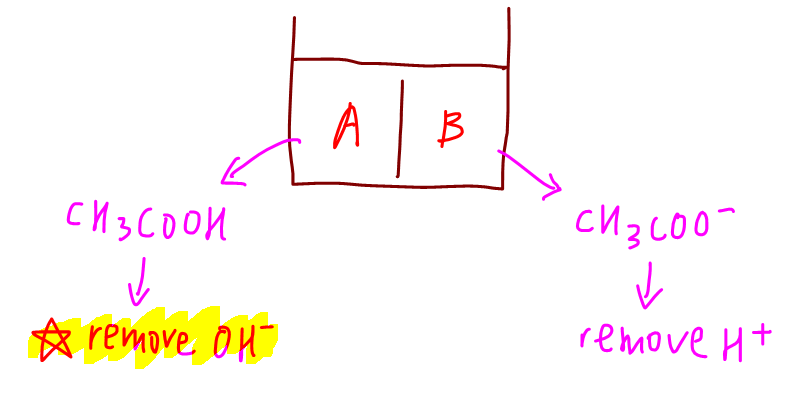
We can use the ICE table to determine the resultant solution.

Notice the final solution is still a mixture of CH3COOH and CH3COO-, hence it remains a buffer.
So we can use the same buffer equation to determine its final pH and the change in pH.
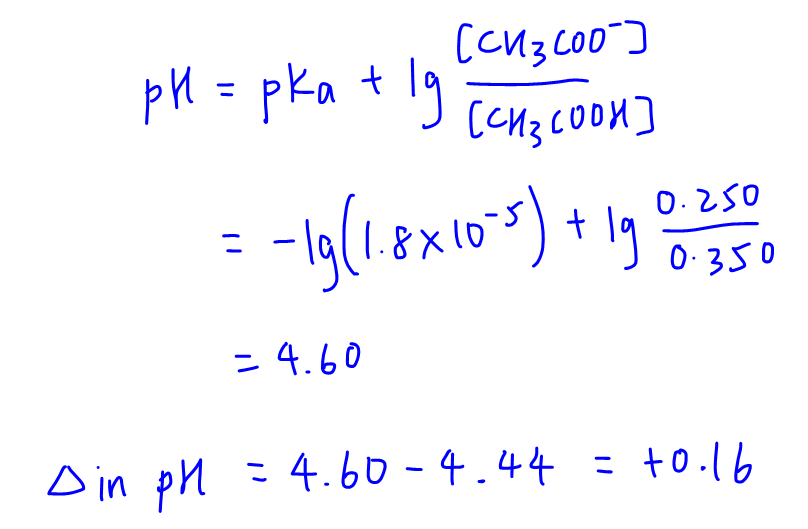
Notice when small and limiting amount of OH- is added, the change in pH is very small.
This is consistent with the definition of a buffer solution, where pH is maintained when small amounts of H+ or OH- is added to it.
Topic: Buffer Solution and Titration Curve, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
